The diverse functions of the DEG/ENaC family: linking genetic and physiological insights
- PMID: 36314992
- PMCID: PMC10148893
- DOI: 10.1113/JP283335
The diverse functions of the DEG/ENaC family: linking genetic and physiological insights
Abstract
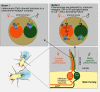
The DEG/ENaC family of ion channels was defined based on the sequence similarity between degenerins (DEG) from the nematode Caenorhabditis elegans and subunits of the mammalian epithelial sodium channel (ENaC), and also includes a diverse array of non-voltage-gated cation channels from across animal phyla, including the mammalian acid-sensing ion channels (ASICs) and Drosophila pickpockets. ENaCs and ASICs have wide ranging medical importance; for example, ENaCs play an important role in respiratory and renal function, and ASICs in ischaemia and inflammatory pain, as well as being implicated in memory and learning. Electrophysiological approaches, both in vitro and in vivo, have played an essential role in establishing the physiological properties of this diverse family, identifying an array of modulators and implicating them in an extensive range of cellular functions, including mechanosensation, acid sensation and synaptic modulation. Likewise, genetic studies in both invertebrates and vertebrates have played an important role in linking our understanding of channel properties to function at the cellular and whole animal/behavioural level. Drawing together genetic and physiological evidence is essential to furthering our understanding of the precise cellular roles of DEG/ENaC channels, with the diversity among family members allowing comparative physiological studies to dissect the molecular basis of these diverse functions.
Keywords: DEG/ENaCs; acid-sensing ion channels; degenerins; epithelial Na+ channels; pickpockets.
© 2022 MRC Laboratory of Molecular Biology. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors have no competing interests to declare.
Figures


References
-
- Adams, C. M. , Snyder, P. M. , & Welsh, M. J. (1999b). Paradoxical stimulation of a DEG/ENaC channel by amiloride. Journal of Biological Chemistry, 274(22), 15500–15504. - PubMed
-
- Ainsley, J. A. , Pettus, J. M. , Bosenko, D. , Gerstein, C. E. , Zinkevich, N. , Anderson, M. G. , Adams, C. M. , Welsh, M. J. , & Johnson, W. A. (2003). Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Current Biology, 13(17), 1557–1563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

