Evaluation of Simvastatin as a Disease-Modifying Treatment for Patients With Parkinson Disease: A Randomized Clinical Trial
- PMID: 36315128
- PMCID: PMC9623477
- DOI: 10.1001/jamaneurol.2022.3718
Evaluation of Simvastatin as a Disease-Modifying Treatment for Patients With Parkinson Disease: A Randomized Clinical Trial
Abstract
Importance: Current treatments manage symptoms of Parkinson disease (PD), but no known treatment slows disease progression. Preclinical and epidemiological studies support the potential use of statins as disease-modifying therapy.
Objective: To determine whether simvastatin has potential as a disease-modifying treatment for patients with moderate PD.
Design, setting, and participants: This randomized clinical trial, a double-blind, parallel-group, placebo-controlled futility trial, was conducted between March 2016 and May 2020 within 23 National Health Service Trusts in England. Participants aged 40 to 90 years with a diagnosis of idiopathic PD, with a modified Hoehn and Yahr stage of 3.0 or less while taking medication, and taking dopaminergic medication with wearing-off phenomenon were included. Data were analyzed from May 2020 to September 2020, with additional analysis in February 2021.
Interventions: Participants were allocated 1:1 to simvastatin or matched placebo via a computer-generated random sequence, stratified by site and Hoehn and Yahr stage. In the simvastatin arm, participants entered a 1-month phase of simvastatin, 40 mg daily, followed by 23 months of simvastatin, 80 mg daily, before a 2-month washout period.
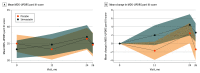
Main outcomes and measures: The prespecified primary outcome was 24-month change in Movement Disorder Society Unified Parkinson Disease Rating Scale (MDS-UPDRS) part III score measured while not taking medication (high scores indicate worse outcome). The primary futility analysis included participants who commenced the 80-mg phase and had valid primary outcome data. The safety analysis included all participants who commenced trial treatment and is reported by dose at time of event.
Results: Of 332 patients assessed for eligibility, 32 declined and 65 were ineligible. Of 235 recruited participants, 97 (41%) were female, 233 (99%) were White, and the mean (SD) age was 65.4 (9.4) years. A total of 216 patients progressed to the 80-mg dose. Primary outcome analysis (n = 178) indicated the simvastatin group had an additional deterioration in MDS-UPDRS III score while not taking medication at 24 months compared with the placebo group (1.52 points; 2-sided 80% CI, -0.77 to 3.80; 1-sided futility test P = .006). A total of 37 serious adverse events (AEs), including 3 deaths, and 171 AEs were reported for participants receiving 0-mg simvastatin; 37 serious AEs and 150 AEs were reported for participants taking 40 mg or 80 mg of simvastatin. Four participants withdrew from the trial because of an AE.
Conclusions and relevance: In this randomized clinical trial, simvastatin was futile as a disease-modifying therapy in patients with PD of moderate severity, providing no evidence to support proceeding to a phase 3 trial.
Trial registration: ISRCTN Identifier: 16108482.
Conflict of interest statement
Figures

References
-
- Sierra S, Ramos MC, Molina P, Esteo C, Vázquez JA, Burgos JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis. 2011;23(2):307-318. doi:10.3233/JAD-2010-101179 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

