Genetic Testing to Inform Epilepsy Treatment Management From an International Study of Clinical Practice
- PMID: 36315135
- PMCID: PMC9623482
- DOI: 10.1001/jamaneurol.2022.3651
Genetic Testing to Inform Epilepsy Treatment Management From an International Study of Clinical Practice
Abstract
Importance: It is currently unknown how often and in which ways a genetic diagnosis given to a patient with epilepsy is associated with clinical management and outcomes.
Objective: To evaluate how genetic diagnoses in patients with epilepsy are associated with clinical management and outcomes.
Design, setting, and participants: This was a retrospective cross-sectional study of patients referred for multigene panel testing between March 18, 2016, and August 3, 2020, with outcomes reported between May and November 2020. The study setting included a commercial genetic testing laboratory and multicenter clinical practices. Patients with epilepsy, regardless of sociodemographic features, who received a pathogenic/likely pathogenic (P/LP) variant were included in the study. Case report forms were completed by all health care professionals.
Exposures: Genetic test results.
Main outcomes and measures: Clinical management changes after a genetic diagnosis (ie, 1 P/LP variant in autosomal dominant and X-linked diseases; 2 P/LP variants in autosomal recessive diseases) and subsequent patient outcomes as reported by health care professionals on case report forms.
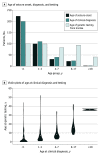
Results: Among 418 patients, median (IQR) age at the time of testing was 4 (1-10) years, with an age range of 0 to 52 years, and 53.8% (n = 225) were female individuals. The mean (SD) time from a genetic test order to case report form completion was 595 (368) days (range, 27-1673 days). A genetic diagnosis was associated with changes in clinical management for 208 patients (49.8%) and usually (81.7% of the time) within 3 months of receiving the result. The most common clinical management changes were the addition of a new medication (78 [21.7%]), the initiation of medication (51 [14.2%]), the referral of a patient to a specialist (48 [13.4%]), vigilance for subclinical or extraneurological disease features (46 [12.8%]), and the cessation of a medication (42 [11.7%]). Among 167 patients with follow-up clinical information available (mean [SD] time, 584 [365] days), 125 (74.9%) reported positive outcomes, 108 (64.7%) reported reduction or elimination of seizures, 37 (22.2%) had decreases in the severity of other clinical signs, and 11 (6.6%) had reduced medication adverse effects. A few patients reported worsening of outcomes, including a decline in their condition (20 [12.0%]), increased seizure frequency (6 [3.6%]), and adverse medication effects (3 [1.8%]). No clinical management changes were reported for 178 patients (42.6%).
Conclusions and relevance: Results of this cross-sectional study suggest that genetic testing of individuals with epilepsy may be materially associated with clinical decision-making and improved patient outcomes.
Conflict of interest statement
Figures



Comment in
-
Genetic Testing in Patients With Epilepsy May Impact Treatments and Improve Outcomes.JAMA Neurol. 2022 Dec 1;79(12):1227-1228. doi: 10.1001/jamaneurol.2022.3391. JAMA Neurol. 2022. PMID: 36315117 No abstract available.
-
From precision diagnosis to precision treatment in epilepsy.Nat Rev Neurol. 2023 Feb;19(2):69-70. doi: 10.1038/s41582-022-00756-0. Nat Rev Neurol. 2023. PMID: 36522546 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

