5-ethyl-2'-deoxyuridine fragilizes Klebsiella pneumoniae outer wall and facilitates intracellular killing by phagocytic cells
- PMID: 36315510
- PMCID: PMC9621411
- DOI: 10.1371/journal.pone.0269093
5-ethyl-2'-deoxyuridine fragilizes Klebsiella pneumoniae outer wall and facilitates intracellular killing by phagocytic cells
Abstract
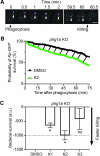
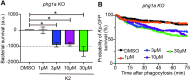
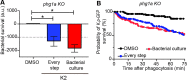
Klebsiella pneumoniae is the causative agent of a variety of severe infections. Many K. pneumoniae strains are resistant to multiple antibiotics, and this situation creates a need for new antibacterial molecules. K. pneumoniae pathogenicity relies largely on its ability to escape phagocytosis and intracellular killing by phagocytic cells. Interfering with these escape mechanisms may allow to decrease bacterial virulence and to combat infections. In this study, we used Dictyostelium discoideum as a model phagocyte to screen a collection of 1,099 chemical compounds. Phg1A KO D. discoideum cells cannot feed upon K. pneumoniae bacteria, unless bacteria bear mutations decreasing their virulence. We identified 3 non-antibiotic compounds that restored growth of phg1A KO cells on K. pneumoniae, and we characterized the mode of action of one of them, 5-ethyl-2'-deoxyuridine (K2). K2-treated bacteria were more rapidly killed in D. discoideum phagosomes than non-treated bacteria. They were more sensitive to polymyxin and their outer membrane was more accessible to a hydrophobic fluorescent probe. These results suggest that K2 acts by rendering the membrane of K. pneumoniae accessible to antibacterial effectors. K2 was effective on three different K. pneumoniae strains, and acted at concentrations as low as 3 μM. K2 has previously been used to treat viral infections but its precise molecular mechanism of action in K. pneumoniae remains to be determined.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
How Phagocytic Cells Kill Different Bacteria: a Quantitative Analysis Using Dictyostelium discoideum.mBio. 2021 Feb 16;12(1):e03169-20. doi: 10.1128/mBio.03169-20. mBio. 2021. PMID: 33593980 Free PMC article.
-
Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes.PLoS One. 2013;8(2):e56847. doi: 10.1371/journal.pone.0056847. Epub 2013 Feb 15. PLoS One. 2013. PMID: 23457627 Free PMC article.
-
Evaluating Different Virulence Traits of Klebsiella pneumoniae Using Dictyostelium discoideum and Zebrafish Larvae as Host Models.Front Cell Infect Microbiol. 2018 Feb 9;8:30. doi: 10.3389/fcimb.2018.00030. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29479519 Free PMC article.
-
The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella pneumoniae.Int J Environ Res Public Health. 2020 Aug 28;17(17):6278. doi: 10.3390/ijerph17176278. Int J Environ Res Public Health. 2020. PMID: 32872324 Free PMC article. Review.
-
Klebsiella pneumoniae: Going on the Offense with a Strong Defense.Microbiol Mol Biol Rev. 2016 Jun 15;80(3):629-61. doi: 10.1128/MMBR.00078-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27307579 Free PMC article. Review.
Cited by
-
Exploring host-pathogen interactions in the Dictyostelium discoideum-Mycobacterium marinum infection model of tuberculosis.Dis Model Mech. 2024 Jul 1;17(7):dmm050698. doi: 10.1242/dmm.050698. Epub 2024 Jul 22. Dis Model Mech. 2024. PMID: 39037280 Free PMC article. Review.
-
Compound K14 inhibits bacterial killing and protease activity in Dictyostelium discoideum phagosomes.PLoS One. 2024 Aug 26;19(8):e0309327. doi: 10.1371/journal.pone.0309327. eCollection 2024. PLoS One. 2024. PMID: 39186559 Free PMC article.
References
-
- Agyeman AA, Bergen PJ, Rao GG, Nation RL, Landersdorfer CB. 2019. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int J Antimicrob Agents 55:105833. doi: 10.1016/j.ijantimicag.2019.10.014 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

