Dietary iron restriction protects against vaso-occlusion and organ damage in murine sickle cell disease
- PMID: 36315910
- PMCID: PMC10023724
- DOI: 10.1182/blood.2022016218
Dietary iron restriction protects against vaso-occlusion and organ damage in murine sickle cell disease
Abstract
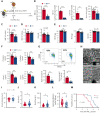
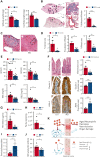
Sickle cell disease (SCD) is an inherited disorder resulting from a β-globin gene mutation, and SCD patients experience erythrocyte sickling, vaso-occlusive episodes (VOE), and progressive organ damage. Chronic hemolysis, inflammation, and repeated red blood cell transfusions in SCD can disrupt iron homeostasis. Patients who receive multiple blood transfusions develop iron overload, and another subpopulation of SCD patients manifest iron deficiency. To elucidate connections between dietary iron, the microbiome, and SCD pathogenesis, we treated SCD mice with an iron-restricted diet (IRD). IRD treatment reduced iron availability and hemolysis, decreased acute VOE, and ameliorated chronic organ damage in SCD mice. Our results extend previous studies indicating that the gut microbiota regulate disease in SCD mice. IRD alters microbiota load and improves gut integrity, together preventing crosstalk between the gut microbiome and inflammatory factors such as aged neutrophils, dampening VOE, and organ damage. These findings provide strong evidence for the therapeutic potential of manipulating iron homeostasis and the gut microbiome to ameliorate SCD pathophysiology. Many treatments, which are under development, focus on lowering the systemic iron concentration to relieve disease complications, and our data suggest that iron-induced changes in microbiota load and gut integrity are related- and novel-therapeutic targets.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no relevant competing financial interests.
Figures

Comment in
-
(De)ironing out sickle cell disease.Blood. 2023 Jan 12;141(2):129-130. doi: 10.1182/blood.2022018791. Blood. 2023. PMID: 36633882 Free PMC article. No abstract available.
References
-
- Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Semin Hematol. 2001;38(1 Suppl 1):30–36. - PubMed
-
- Koduri PR. Iron in sickle cell disease: a review why less is better. Am J Hematol. 2003;73(1):59–63. - PubMed
-
- Castro O, Haddy TB. Improved survival of iron-deficient patients with sickle erythrocytes. N Engl J Med. 1983;308(9):527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

