Clinical and cost effectiveness of single stage compared with two stage revision for hip prosthetic joint infection (INFORM): pragmatic, parallel group, open label, randomised controlled trial
- PMID: 36316046
- PMCID: PMC9645409
- DOI: 10.1136/bmj-2022-071281
Clinical and cost effectiveness of single stage compared with two stage revision for hip prosthetic joint infection (INFORM): pragmatic, parallel group, open label, randomised controlled trial
Erratum in
-
Clinical and cost effectiveness of single stage compared with two stage revision for hip prosthetic joint infection (INFORM): pragmatic, parallel group, open label, randomised controlled trial.BMJ. 2022 Dec 6;379:o2924. doi: 10.1136/bmj.o2924. BMJ. 2022. PMID: 36740865 No abstract available.
Abstract
Objectives: To determine whether patient reported outcomes improve after single stage versus two stage revision surgery for prosthetic joint infection of the hip, and to determine the cost effectiveness of these procedures.
Design: Pragmatic, parallel group, open label, randomised controlled trial.
Setting: High volume tertiary referral centres or orthopaedic units in the UK (n=12) and in Sweden (n=3), recruiting from 1 March 2015 to 19 December 2018.
Participants: 140 adults (aged ≥18 years) with a prosthetic joint infection of the hip who required revision (65 randomly assigned to single stage and 75 to two stage revision).
Interventions: A computer generated 1:1 randomisation list stratified by hospital was used to allocate participants with prosthetic joint infection of the hip to a single stage or a two stage revision procedure.
Main outcome measures: The primary intention-to-treat outcome was pain, stiffness, and functional limitations 18 months after randomisation, measured by the Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC) score. Secondary outcomes included surgical complications and joint infection. The economic evaluation (only assessed in UK participants) compared quality adjusted life years and costs between the randomised groups.
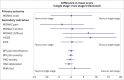
Results: The mean age of participants was 71 years (standard deviation 9) and 51 (36%) were women. WOMAC scores did not differ between groups at 18 months (mean difference 0.13 (95% confidence interval -8.20 to 8.46), P=0.98); however, the single stage procedure was better at three months (11.53 (3.89 to 19.17), P=0.003), but not from six months onwards. Intraoperative events occurred in five (8%) participants in the single stage group and 20 (27%) in the two stage group (P=0.01). At 18 months, nine (14%) participants in the single stage group and eight (11%) in the two stage group had at least one marker of possible ongoing infection (P=0.62). From the perspective of healthcare providers and personal social services, single stage revision was cost effective with an incremental net monetary benefit of £11 167 (95% confidence interval £638 to £21 696) at a £20 000 per quality adjusted life years threshold (£1.0; $1.1; €1.4).
Conclusions: At 18 months, single stage revision compared with two stage revision for prosthetic joint infection of the hip showed no superiority by patient reported outcome. Single stage revision had a better outcome at three months, fewer intraoperative complications, and was cost effective. Patients prefer early restoration of function, therefore, when deciding treatment, surgeons should consider patient preferences and the cost effectiveness of single stage surgery.
Trial registration: ISRCTN registry ISRCTN10956306.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from NIHR for the submitted work. RGH reports individual payments as co-chair, UK Committee on Research Integrity. SM reports payments for organising the Palacademy Basic Sciences Course for Fellowship of the Royal College of Surgeons trainees by Heraeus. OR reports research funding paid to employer by Pfizer, educational/lecturer payments from Link Sweden, payments for Novartis advisory board, and unpaid roles as past president, International Society of Arthroplasty Registries, and deputy editor, Clinical Orthopaedics and Related Research. MW reports an institutional payment for an institutional contract between Exeter Hip Unit and Stryker Orthopaedics, personal payments in relation to intellectual property for Exeter Hip System, patents in relation to Exeter Hip Systems, honorary treasurer (British Hip Society), and associate editor (Annals of the Royal College of Surgeons of England). MRW is principal investigator of the Lot 2 contract for statistical analysis and support for the National Joint Registry from Healthcare Quality Improvement Partnership, principal investigator on an independently conducted research grant, funded by CeramTec investigating the association of total hip replacement bearing materials with outcomes, editor of two general orthopaedic textbooks for which he receives royalties from Taylor and Francis, undertakes teaching on basic sciences for Orthopaedic trainees preparing for the for Fellowship of the Royal College of Surgeons and on courses on the principles and performance of total hip replacements for trainees with market rate institutional payment for this teaching from Heraeus, editorial board member of Bone Joint Journal and Hip International, and research committee member of British Orthopaedic Association and British Hip Society.
Figures


References
-
- National Joint Registry. 17th annual report Hemel Hempstead: NJR Service Centre 2020. Available from: https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2017th%20Ann... Accessed on 14th March 2022.
-
- Scottish Arthroplasty Project. Annual report 2020 Edinburgh: Public Health Scotland; 2020. Available from: https://readymag.com/PHIDigital/SAP-Annual-Report-2020/. Accessed on 14th March 2022.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
