First-in-human phase I/II, open-label study of the anti-OX40 agonist INCAGN01949 in patients with advanced solid tumors
- PMID: 36316061
- PMCID: PMC9628691
- DOI: 10.1136/jitc-2021-004235
First-in-human phase I/II, open-label study of the anti-OX40 agonist INCAGN01949 in patients with advanced solid tumors
Abstract
Background: OX40 is a costimulatory receptor upregulated on antigen-activated T cells and constitutively expressed on regulatory T cells (Tregs). INCAGN01949, a fully human immunoglobulin G1κ anti-OX40 agonist monoclonal antibody, was designed to promote tumor-specific immunity by effector T-cell activation and Fcγ receptor-mediated Treg depletion. This first-in-human study was conducted to determine the safety, tolerability, and preliminary efficacy of INCAGN01949.
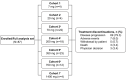
Methods: Phase I/II, open-label, non-randomized, dose-escalation and dose-expansion study conducted in patients with advanced or metastatic solid tumors. Patients received INCAGN01949 monotherapy (7-1400 mg) in 14-day cycles while deriving benefit. Safety measures, clinical activity, pharmacokinetics, and pharmacodynamic effects were assessed and summarized with descriptive statistics.
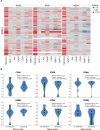
Results: Eighty-seven patients were enrolled; most common tumor types were colorectal (17.2%), ovarian (8.0%), and non-small cell lung (6.9%) cancers. Patients received a median three (range 1-9) prior therapies, including immunotherapy in 24 patients (27.6%). Maximum tolerated dose was not reached; one patient (1.1%) receiving 350 mg dose reported dose-limiting toxicity of grade 3 colitis. Treatment-related adverse events were reported in 45 patients (51.7%), with fatigue (16 (18.4%)), rash (6 (6.9%)), and diarrhea (6 (6.9%)) being most frequent. One patient (1.1%) with metastatic gallbladder cancer achieved a partial response (duration of 6.3 months), and 23 patients (26.4%) achieved stable disease (lasting >6 months in one patient). OX40 receptor occupancy was maintained over 90% among all patients receiving doses of ≥200 mg, while no treatment-emergent antidrug antibodies were detected across all dose levels. Pharmacodynamic results demonstrated that treatment with INCAGN01949 did not enhance proliferation or activation of T cells in peripheral blood or reduce circulating Tregs, and analyses of tumor biopsies did not demonstrate any consistent increase in effector T-cell infiltration or function, or decrease in infiltrating Tregs.
Conclusion: No safety concerns were observed with INCAGN01949 monotherapy in patients with metastatic or advanced solid tumors. However, tumor responses and pharmacodynamic effects on T cells in peripheral blood and post-therapy tumor biopsies were limited. Studies evaluating INCAGN01949 in combination with other therapies are needed to further evaluate the potential of OX40 agonism as a therapeutic approach in patients with advanced solid tumors.
Trial registration number: NCT02923349.
Keywords: Clinical Trials as Topic; Immunomodulation; Lymphocytes, Tumor-Infiltrating; T-Lymphocytes; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: EJD reports research funding (institution) from Actuate, BMS, FivePrime, Genentech, Incyte, Karyopharm and TopAlliance Biosciences; honoraria from MJH Life Sciences; and advisory role for Deciphera. JM-L reports lecture fees from Astellas, Bristol-Myers Squibb, MSD, Novartis, Pierre Fabre, Pfizer, Roche and Sanofi; advisory fees from Bristol-Myers Squibb, Highlight Therapeutics, Novartis, Pierre Fabre, Roche and Sanofi; research grants from Sanofi; travel grants from Bristol-Myers Squibb, Ipsen, MSD, Novartis, Pierre Fabre, Pfizer and Roche. RK reports grants from MSD, Clovis and MSD; advisory role for Basilea, PharmaMar; advisory fees from AstraZeneca, Clovis, Eisai, GSK, Incyte, iTEOS, Pfizer and Roche. DCC reports advisory role for HUYA, Nektar Therapeutics, Pfizer and Werewolf Pharmaceuticals. SPB reports research funding and honoraria from Nucana plc; advisory fees from Amphista and Ellipses; clinical trials funding from Astex, MSD, Redx Pharma, Roche and UCB. DB reports no competing interests. DBC reports advisory role with AbbVie and Rafael Pharmaceuticals; honoraria from OncLive/MJH Life Sciences; research funding (institution) from Advaxis, Array BioPharma, Bristol-Myers Squibb, Celgene, Corcept Therapeutics, EMD Serono, Incyte, Lilly, Rafael Pharmaceuticals and Synta; and travel, accommodations and expenses from AbbVie. MV reports advisory role with Debiopharm and Roche; and travel from Roche. REM reports advisory fees from AZD, Clovis Oncology, Ellipses, GSK, Merck and Shigoni; speakers' fees from AZD, Clovis Oncology, GSK and Roche. PHD and AO report no competing interests. KS reports advisory fees from QED Therapeutics. JEJ, JC and TC report former employment and stock ownership with Incyte. JP and XC report employment and stock ownership with Incyte. JMM reports grants from Amgen, AstraZeneca, Bristol-Myers Squibb, EMD Serono, Immunocore, Incyte, Macrogenics, Merck Sharp & Dohme, Novartis, Polynoma and Sanofi; advisory role with Amgen and Merck Sharp & Dohme; honoraria from EMD Serono and Pfizer; advisory board for Array BioPharma, Bristol-Myers Squibb, Eisai/Merck, EMD Serono, Sanofi/Regeneron and Seagen.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
