Transition to rilonacept monotherapy from oral therapies in patients with recurrent pericarditis
- PMID: 36316102
- PMCID: PMC9887401
- DOI: 10.1136/heartjnl-2022-321328
Transition to rilonacept monotherapy from oral therapies in patients with recurrent pericarditis
Erratum in
-
Correction: Transition to rilonacept monotherapy from oral therapies in patients with recurrent pericarditis.Heart. 2023 Dec 20;110(2):e1. doi: 10.1136/heartjnl-2022-321328corr1. Heart. 2023. PMID: 38123179 Free PMC article. No abstract available.
Abstract
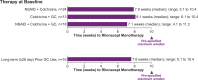
Objective: Polypharmacy management of recurrent pericarditis (RP) often involves long-term therapies, often with negative effects. Slow tapering of oral therapies is often required to avoid recurrence. A post hoc analysis of the phase III trial Rilonacept inHibition of interleukin-1 Alpha and beta for recurrent Pericarditis: a pivotal Symptomatology and Outcomes Study (RHAPSODY) evaluated investigator approaches to transitioning to IL-1 blockade monotherapy with rilonacept, which was hypothesised to allow accelerated withdrawal of common multidrug pericarditis regimens.
Methods: RHAPSODY was a multicentre (Australia, Israel, Italy, USA), double-blind, placebo-controlled, randomised-withdrawal trial in adults and adolescents with RP. Investigators initiated rilonacept at the labelled dose level and discontinued oral pericarditis therapies during the 12-week run-in; randomised patients received study drug as monotherapy. Time to rilonacept monotherapy was quantified in patients receiving multidrug regimens at baseline who achieved rilonacept monotherapy during run-in.
Results: In 86 enrolled patients, mean time to rilonacept monotherapy was 7.9 weeks, with no recurrences. Of these, 64% (n=55) entered on multidrug regimens: non-steroidal anti-inflammatory drugs (NSAIDs) plus colchicine (44% (24/55)), colchicine plus glucocorticoids (24% (13/55)), or NSAIDs, colchicine, plus glucocorticoids (33% (18/55)). Investigators transitioned patients receiving colchicine and glucocorticoids at baseline to rilonacept monotherapy without recurrence regardless of taper approach: sequential (n=14; median, 7.7 weeks) or concurrent (n=17; median, 8.0 weeks). Median time to rilonacept monotherapy was similar regardless of glucocorticoid dose and duration: ≤15 mg/day (n=21): 7.3 weeks; >15 mg/day (n=18): 8.0 weeks; long-term (≥28 days): 7.6 weeks.
Conclusions: Rapid discontinuation of oral RP therapies while transitioning to rilonacept monotherapy was feasible without triggering pericarditis recurrence.
Trial registration number: NCT03737110.
Keywords: pericarditis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AB reports institutional funding from Kiniksa Pharmaceuticals as an investigative site; an unrestricted research grant from Sobi and Acarpia; and travel and accommodation for advisory committee from Sobi and Kiniksa Pharmaceuticals. AW reports personal fees from Kiniksa Pharmaceuticals during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. AW is a former employee of Kiniksa Pharmaceuticals; the work here was completed during his employment. SAL reports consultant fees for Kiniksa Pharmaceuticals, Sobi Pharmaceuticals and Medtronic. AA reports grants and personal fees from Kiniksa Pharmaceuticals during the conduct of the study; grants and personal fees from Olatec, Serpin, Novartis and Janssen; and personal fees from Novo-Nordisk and Cromos Pharma outside the submitted work. PCC reports grants and personal fees from Kiniksa Pharmaceuticals; grants from Novartis Pharmaceuticals; and personal fees from Sobi Pharmaceuticals outside the submitted work. LZ reports personal fees and other from Kiniksa Pharmaceuticals outside the submitted work. ML reports grants and advisory board, consulting, and other fees from Kiniksa Pharmaceuticals outside the submitted work and consulting fees from Sobi Pharmaceuticals outside the submitted work. BSL reports personal fees from Kiniksa Pharmaceuticals during the conduct of the study. DL reports personal fees from Regeneron outside the submitted work. SN reports grants and personal fees from Kiniksa Pharmaceuticals during the conduct of the study; grants and personal fees from AstraZeneca, Anthera, Resverlogix, Sanofi-Regeneron and Esperion; personal fees from Akcea, Eli Lilly, Omthera, Merck, Takeda, CSL Behring and Boehringer Ingelheim; and grants from Amgen, Novartis, Cerenis, The Medicines Company, Liposcience, Roche and InfraReDx outside the submitted work. ALK reports grants and other from Kiniksa Pharmaceuticals during the conduct of the study and other fees from Cardiol Therapeutics, Sobi Pharmaceuticals and Pfizer Pharmaceuticals outside the submitted work. MI reports other fees from Kiniksa Pharmaceuticals and Sobi outside the submitted work. JFP reports personal fees and other from Kiniksa Pharmaceuticals outside the submitted work and is a patent inventor on pending patent applications licensed to Kiniksa Pharmaceuticals covering methods of using rilonacept for treating recurrent pericarditis.
Figures


Comment in
-
Recurrent pericarditis: moving from the middle ages to renaissance.Heart. 2023 Jan 27;109(4):250-252. doi: 10.1136/heartjnl-2022-321749. Heart. 2023. PMID: 36316101 No abstract available.
References
-
- Adler Y, Charron P, Imazio M, et al. . 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–64. 10.1093/eurheartj/ehv318 - DOI - PMC - PubMed
