Endoscopic Findings and Treatment of Gastric Neoplasms in Familial Adenomatous Polyposis
- PMID: 36316112
- PMCID: PMC9633932
- DOI: 10.5230/jgc.2022.22.e30
Endoscopic Findings and Treatment of Gastric Neoplasms in Familial Adenomatous Polyposis
Abstract
Purpose: Gastric neoplasia is a common manifestation of familial adenomatous polyposis (FAP). This study aimed to elucidate the clinical characteristics, endoscopic features including fundic gland polyposis (FGPsis), and treatment outcomes of gastric neoplasms (GNs) in patients with FAP.
Materials and methods: A total of 35 patients diagnosed with FAP, including nine patients from four pedigrees who underwent esophagogastroduodenoscopy (EGD), were investigated regarding patient characteristics, GN morphology, and treatment outcomes.
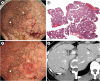
Results: Twenty-one patients (60.0%) had 38 GNs; 33 (86.8%) and 5 (13.2%) were histologically diagnosed with adenocarcinoma and adenoma, respectively. There were no specific patient characteristics related to GNs. Nodule-type GNs were more prevalent in patients with FGP than without (52.2% vs. 0.0%, P=0.002) in the upper body of the stomach. Conversely, depressed-type GNs were fewer in patients with FGPsis than in those without (13.0% vs. 73.3%, P<0.001). Slightly elevated-type GNs were observed in both groups (34.8% vs. 20.0%, P=0.538). Even within pedigrees, the background gastric mucosa and types of GNs varied. In total, 24 GNs were treated with endoscopic submucosal dissection (ESD) and eight with endoscopic mucosal resection (EMR). EMR was selected for GNs with FGPsis because of the technical difficulty of ESD, resulting in a lower en bloc resection rate (62.5% vs. 100%, P=0.014).
Conclusions: Our study indicates the necessity of routine EGD surveillance in patients diagnosed with FAP. Notably, the morphology and location of GNs differed between patients with and without FGPsis. Endoscopic treatment and outcomes require more attention in cases of FGPsis.
Keywords: Endoscopic mucosal resections; Endoscopic submucosal dissections; Familial adenomatous polyposis; Gastric neoplasms.
Copyright © 2022. Korean Gastric Cancer Association.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


References
-
- Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–713. - PubMed
-
- Dinarvand P, Davaro EP, Doan JV, Ising ME, Evans NR, Phillips NJ, et al. Familial adenomatous polyposis syndrome: an update and review of extraintestinal manifestations. Arch Pathol Lab Med. 2019;143:1382–1398. - PubMed
-
- Clark S, Hyer W, Guenther T. Familial adenomatous polyposis. J Pediatr Surg. 2007;42:1463–1464. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

