Photo-crosslinked lignin/PAN electrospun separator for safe lithium-ion batteries
- PMID: 36316362
- PMCID: PMC9622728
- DOI: 10.1038/s41598-022-23038-7
Photo-crosslinked lignin/PAN electrospun separator for safe lithium-ion batteries
Abstract
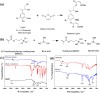
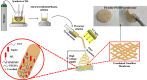
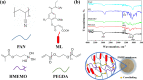
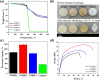
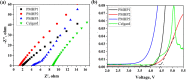
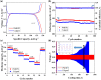
A novel crosslinked electrospun nanofibrous membrane with maleated lignin (ML) and poly(acrylonitrile) (PAN) is presented as a separator for lithium-ion batteries (LIBs). Alkali lignin was treated with an esterification agent of maleic anhydride, resulting in a substantial hydroxyl group conversion to enhance the reactivity and mechanical properties of the final nanofiber membranes. The maleated lignin (ML) was subsequently mixed with UV-curable formulations (up to 30% wt) containing polyethylene glycol diacrylate (PEGDA), hydrolyzed 3-(Trimethoxysilyl)propyl methacrylate (HMEMO) as crosslinkers, and poly(acrylonitrile) (PAN) as a precursor polymer. UV-electrospinning was used to fabricate PAN/ML/HMEMO/PEGDA (PMHP) crosslinked membranes. PMHP membranes made of electrospun nanofibers feature a three-dimensional (3D) porous structure with interconnected voids between the fibers. The mechanical strength of PMHP membranes with a thickness of 25 µm was enhanced by the variation of the cross-linkable formulations. The cell assembled with PMHP2 membrane (20 wt% of ML) showed the maximum ionic conductivity value of 2.79*10-3 S cm-1, which is significantly higher than that of the same cell with the liquid electrolyte and commercial Celgard 2400 (6.5*10-4 S cm-1). The enhanced LIB efficiency with PMHP2 membrane can be attributed to its high porosity, which allows better electrolyte uptake and demonstrates higher ionic conductivity. As a result, the cell assembled with LiFePO4 cathode, Li metal anode, and PMHP2 membrane had a high initial discharge specific capacity of 147 mAh g-1 at 0.1 C and exhibited outstanding rate performance. Also, it effectively limits the formation of Li dendrites over 1000 h. PMHP separators have improved chemical and physical properties, including porosity, thermal, mechanical, and electrochemical characteristics, compared with the commercial ones.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Koohi-Fayegh S, Rosen MA. A review of energy storage types, applications and recent developments. J. Energy Storage. 2020;27:101047. doi: 10.1016/j.est.2019.101047. - DOI
-
- Ge J, et al. Self-deformed Si/Graphene@C anode for stress relief in lithium ion batteries. Mater. Today Sustain. 2022;19:100165. doi: 10.1016/J.MTSUST.2022.100165. - DOI
-
- Ue M, Uosaki K. Recent progress in liquid electrolytes for lithium metal batteries. Curr. Opin. Electrochem. 2019;17:106–113. doi: 10.1016/j.coelec.2019.05.001. - DOI
-
- Tolganbek N, Yerkinbekova Y, Kalybekkyzy S, Bakenov Z, Mentbayeva A. Current state of high voltage olivine structured LiMPO4 cathode materials for energy storage applications: A review. J. Alloys Compd. 2021;882:160774. doi: 10.1016/j.jallcom.2021.160774. - DOI
-
- Tolganbek N, et al. Interface modification of NASICON-type Li-ion conducting ceramic electrolytes: A critical evaluation. Mater. Adv. 2022;3(7):3055–3069. doi: 10.1039/d1ma01239h. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

