Persistent muscle hyperalgesia after adolescent stress is exacerbated by a mild-nociceptive input in adulthood and is associated with microglia activation
- PMID: 36316425
- PMCID: PMC9622712
- DOI: 10.1038/s41598-022-21808-x
Persistent muscle hyperalgesia after adolescent stress is exacerbated by a mild-nociceptive input in adulthood and is associated with microglia activation
Abstract
Non-specific low back pain (LBP) is a major global disease burden and childhood adversity predisposes to its development. The mechanisms are largely unknown. Here, we investigated if adversity in young rats augments mechanical hyperalgesia and how spinal cord microglia contribute to this. Adolescent rats underwent restraint stress, control animals were handled. In adulthood, all rats received two intramuscular injections of NGF/saline or both into the lumbar multifidus muscle. Stress induced in rats at adolescence lowered low back pressure pain threshold (PPT; p = 0.0001) and paw withdrawal threshold (PWT; p = 0.0007). The lowered muscle PPT persisted throughout adulthood (p = 0.012). A subsequent NGF in adulthood lowered only PPT (d = 0.87). Immunohistochemistry revealed changes in microglia morphology: stress followed by NGF induced a significant increase in ameboid state (p < 0.05). Repeated NGF injections without stress showed significantly increased cell size in surveilling and bushy states (p < 0.05). Thus, stress in adolescence induced persistent muscle hyperalgesia that can be enhanced by a mild-nociceptive input. The accompanying morphological changes in microglia differ between priming by adolescent stress and by nociceptive inputs. This novel rodent model shows that adolescent stress is a risk factor for the development of LBP in adulthood and that morphological changes in microglia are signs of spinal mechanisms involved.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




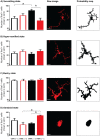

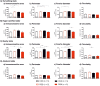
References
-
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: The IASP classification of chronic pain for the international classification of diseases (ICD-11) Pain. 2019;160(1):19–27. doi: 10.1097/j.pain.0000000000001384. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

