Pan-vaccine analysis reveals innate immune endotypes predictive of antibody responses to vaccination
- PMID: 36316476
- PMCID: PMC9747610
- DOI: 10.1038/s41590-022-01329-5
Pan-vaccine analysis reveals innate immune endotypes predictive of antibody responses to vaccination
Abstract
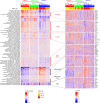
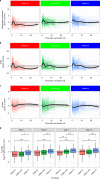
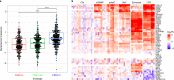
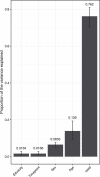
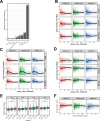
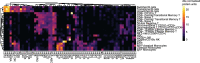
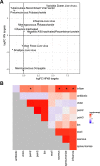
Several studies have shown that the pre-vaccination immune state is associated with the antibody response to vaccination. However, the generalizability and mechanisms that underlie this association remain poorly defined. Here, we sought to identify a common pre-vaccination signature and mechanisms that could predict the immune response across 13 different vaccines. Analysis of blood transcriptional profiles across studies revealed three distinct pre-vaccination endotypes, characterized by the differential expression of genes associated with a pro-inflammatory response, cell proliferation, and metabolism alterations. Importantly, individuals whose pre-vaccination endotype was enriched in pro-inflammatory response genes known to be downstream of nuclear factor-kappa B showed significantly higher serum antibody responses 1 month after vaccination. This pro-inflammatory pre-vaccination endotype showed gene expression characteristic of the innate activation state triggered by Toll-like receptor ligands or adjuvants. These results demonstrate that wide variations in the transcriptional state of the immune system in humans can be a key determinant of responsiveness to vaccination.
© 2022. The Author(s).
Conflict of interest statement
O.L. is a named inventor on patents held by Boston Children’s Hospital regarding human in vitro systems modeling vaccine action and vaccine adjuvants. B.P. serves on the External Immunology Network of GSK, and on the scientific advisory board of Medicago, CircBio, Sanofi, EdJen and Boehringer-Ingelheim. S.H.K. receives consulting fees from Northrop Grumman and Peraton. T.H. owns stock in GSK and Pfizer. The remaining authors declare no competing interests.
Figures





Comment in
-
Immune signature atlas of vaccines: learning from the good responders.Nat Immunol. 2022 Dec;23(12):1654-1656. doi: 10.1038/s41590-022-01361-5. Nat Immunol. 2022. PMID: 36443516 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

