Potential Cardiovascular Events Avoided with Bempedoic Acid Plus Ezetimibe Fixed-Dose Combination Compared with Ezetimibe Alone in Patients with Atherosclerotic Cardiovascular Disease Taking Maximally Tolerated Statins
- PMID: 36316612
- PMCID: PMC9845167
- DOI: 10.1007/s40256-022-00552-7
Potential Cardiovascular Events Avoided with Bempedoic Acid Plus Ezetimibe Fixed-Dose Combination Compared with Ezetimibe Alone in Patients with Atherosclerotic Cardiovascular Disease Taking Maximally Tolerated Statins
Abstract
Background: Patients with atherosclerotic cardiovascular disease who require additional low-density lipoprotein cholesterol (LDL-C) lowering despite maximally tolerated statins have a significant unmet medical need and are at increased risk of future cardiovascular events and a reduced quality of life.
Objective: We aimed to estimate the percentage of cardiovascular events avoided following treatment with a fixed-dose combination of bempedoic acid plus ezetimibe (BA+EZE FDC) versus ezetimibe (EZE) in patients with atherosclerotic cardiovascular disease receiving maximally tolerated statins across a range of baseline LDL-C levels.
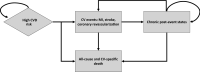
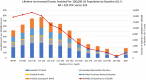
Methods: A Markov cohort simulation model estimated major adverse cardiovascular events avoided over a lifetime horizon among patients with atherosclerotic cardiovascular disease and baseline LDL-C levels from 80 to >200 mg/dL. BA+EZE FDC was compared with EZE based on mean percent LDL-C reductions versus placebo reported in a phase III trial. Health outcomes for the average patient were extrapolated to a US population of 100,000 persons using evidence on contemporary LDL-C levels from the National Health and Nutrition Examination Survey.
Results: Among patients with atherosclerotic cardiovascular disease not at the LDL-C goal with maximally tolerated statins, the addition of BA+EZE FDC compared with the addition of EZE was predicted to provide incremental absolute reductions in major adverse cardiovascular events dependent on baseline LDL-C levels at the population level. For those with baseline LDL-C of 101-110 mg/dL (n = 15,237), there were 4.9% (744) fewer events predicted, while for patients with baseline LDL-C of > 200 mg/dL (n = 1689), 10.9% (184) fewer events were predicted through the addition of BA+EZE FDC versus EZE.
Conclusions: Further LDL-C reductions through the addition of BA+EZE FDC to maximally tolerated statins are predicted to reduce major adverse cardiovascular events compared with the addition of EZE. Benefits are potentially greater among those with higher starting LDL-C.
© 2022. The Author(s).
Conflict of interest statement
R. Brett McQueen has received institutional funding to the University of Colorado from Real Endpoints, LLC. Seth J. Baum has received funding and/or consulting fees from Amgen, AstraZeneca, Axcella, Boehringer Ingelheim, Esperion, Lilly, Madrigal, and Novartis. Michael J. Louie is an employee of Esperion Therapeutics, Inc., and may own Esperion stock or stock options. William J. Sasiela is a former (retired) employee of and a current consultant for Esperion Therapeutics, Inc., and may own Esperion stock or stock options. Aikaterini Bilitou is an employee of Daiichi Sankyo Europe, GmbH, which has a corporate agreement with Esperion Therapeutics, Inc. Hemal Shah is an employee of Value Matters, LLC, and a consultant for Esperion Therapeutics, Inc. Beth Nash is a former employee and consultant of Real Endpoints, which received financial support for this study from Esperion Therapeutics, Inc. Kristin K. Gillard is an employee of Esperion Therapeutics and may own Esperion stock or stock options. Kausik K. Ray has received research grants from Amgen, Daiichi Sankyo, MSD, Pfizer, Regeneron, and Sanofi, and honoraria or consulting fees from Abbvie, Algorithm, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Cerenis, Cipla, Esperion, IONIS, Kowa, Lilly, Medicines Company, MSD, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Resverlogix, Sanofi, Silence Therapeutics, and Takeda.
Figures
Similar articles
-
Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy.Eur J Prev Cardiol. 2020 Apr;27(6):593-603. doi: 10.1177/2047487319864671. Epub 2019 Jul 29. Eur J Prev Cardiol. 2020. PMID: 31357887 Free PMC article. Clinical Trial.
-
Effect of bempedoic acid plus ezetimibe fixed-dose combination vs ezetimibe or placebo on low-density lipoprotein cholesterol in patients with type 2 diabetes and hypercholesterolemia not treated with statins.Am J Prev Cardiol. 2021 Oct 4;8:100278. doi: 10.1016/j.ajpc.2021.100278. eCollection 2021 Dec. Am J Prev Cardiol. 2021. PMID: 34746903 Free PMC article.
-
Efficacy and safety of bempedoic acid in patients not receiving statins in phase 3 clinical trials.J Clin Lipidol. 2022 May-Jun;16(3):286-297. doi: 10.1016/j.jacl.2022.03.001. Epub 2022 Mar 13. J Clin Lipidol. 2022. PMID: 35346603 Clinical Trial.
-
Role of Bempedoic Acid in Clinical Practice.Cardiovasc Drugs Ther. 2021 Aug;35(4):853-864. doi: 10.1007/s10557-021-07147-5. Epub 2021 Apr 5. Cardiovasc Drugs Ther. 2021. PMID: 33818688 Free PMC article. Review.
-
Bempedoic Acid: A Review in Cardiovascular Risk Reduction in Statin-Intolerant Patients.Am J Cardiovasc Drugs. 2025 Jan;25(1):7-16. doi: 10.1007/s40256-024-00714-9. Epub 2025 Jan 23. Am J Cardiovasc Drugs. 2025. PMID: 39847220 Review.
Cited by
-
Safety and efficacy of bempedoic acid among patients with statin intolerance and those without: A meta-analysis and a systematic randomized controlled trial review.PLoS One. 2024 Jan 26;19(1):e0297854. doi: 10.1371/journal.pone.0297854. eCollection 2024. PLoS One. 2024. PMID: 38277431 Free PMC article.
-
Hypercholesterolemia: a literature review on management using tafolecimab: a novel member of PCSK9 monoclonal antibodies.Ann Med Surg (Lond). 2024 Mar 12;86(5):2818-2827. doi: 10.1097/MS9.0000000000001945. eCollection 2024 May. Ann Med Surg (Lond). 2024. PMID: 38694324 Free PMC article. Review.
-
Addition of Bempedoic Acid to Statin-Ezetimibe versus Statin Titration in Patients with High Cardiovascular Risk: A Single-Centre Prospective Study.J Cardiovasc Dev Dis. 2024 Sep 14;11(9):286. doi: 10.3390/jcdd11090286. J Cardiovasc Dev Dis. 2024. PMID: 39330344 Free PMC article.
-
Risk of cardiovascular outcomes with bempedoic acid in high-risk statin intolerant patients: a systematic review and meta analysis.Future Cardiol. 2024;20(11-12):639-650. doi: 10.1080/14796678.2024.2388478. Epub 2024 Aug 14. Future Cardiol. 2024. PMID: 39140596 Free PMC article.
-
A Systematic Review and Meta-Analysis on the Role of Bempedoic Acid in Cardiovascular Outcomes for Patients With Statin Intolerance.Cureus. 2024 Jun 3;16(6):e61572. doi: 10.7759/cureus.61572. eCollection 2024 Jun. Cureus. 2024. PMID: 38962583 Free PMC article. Review.
References
-
- World Health Organization. Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...). Accessed 15 Feb 2019.
-
- American Heart Association. Cardiovascular disease: a costly burden for America. Projections through 2035. 2017. https://www.heart.org/-/media/files/get-involved/advocacy/burden-report-.... Accessed 15 Feb 2019.
-
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

