Developmentally regulated mitochondrial biogenesis and cell death competence in maize pollen
- PMID: 36316635
- PMCID: PMC9624016
- DOI: 10.1186/s12870-022-03897-y
Developmentally regulated mitochondrial biogenesis and cell death competence in maize pollen
Abstract
Background: Cytoplasmic male sterility (CMS) is a maternally inherited failure to produce functional pollen that most commonly results from expression of novel, chimeric mitochondrial genes. In Zea mays, cytoplasmic male sterility type S (CMS-S) is characterized by the collapse of immature, bi-cellular pollen. Molecular and cellular features of developing CMS-S and normal (N) cytoplasm pollen were compared to determine the role of mitochondria in these differing developmental fates.
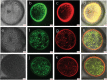
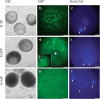
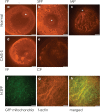
Results: Terminal deoxynucleotidyl transferase dUTP nick end labeling revealed both chromatin and nuclear fragmentation in the collapsed CMS-S pollen, demonstrating a programmed cell death (PCD) event sharing morphological features with mitochondria-signaled apoptosis in animals. Maize plants expressing mitochondria-targeted green fluorescent protein (GFP) demonstrated dynamic changes in mitochondrial morphology and association with actin filaments through the course of N-cytoplasm pollen development, whereas mitochondrial targeting of GFP was lost and actin filaments were disorganized in developing CMS-S pollen. Immunoblotting revealed significant developmental regulation of mitochondrial biogenesis in both CMS-S and N mito-types. Nuclear and mitochondrial genome encoded components of the cytochrome respiratory pathway and ATP synthase were of low abundance at the microspore stage, but microspores accumulated abundant nuclear-encoded alternative oxidase (AOX). Cytochrome pathway and ATP synthase components accumulated whereas AOX levels declined during the maturation of N bi-cellular pollen. Increased abundance of cytochrome pathway components and declining AOX also characterized collapsed CMS-S pollen. The accumulation and robust RNA editing of mitochondrial transcripts implicated translational or post-translational control for the developmentally regulated accumulation of mitochondria-encoded proteins in both mito-types.
Conclusions: CMS-S pollen collapse is a PCD event coincident with developmentally programmed mitochondrial events including the accumulation of mitochondrial respiratory proteins and declining protection against mitochondrial generation of reactive oxygen species.
Keywords: Cytoplasmic male sterility; Maize; Mitochondria; Pollen development; Programmed cell death.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Carlsson J, Leino M. Sohlberg J Sundstrom JF, Glimelius K. Mitochondrial regulation of flower development Mitochondrion. 2008;8:74–86. - PubMed
MeSH terms
Substances
Grants and funding
- IOS-0816782/National Science Foundation
- IOS-0816782/National Science Foundation
- IOS-0816782/National Science Foundation
- IOS-0816782/National Science Foundation
- IOS-0816782/National Science Foundation
- IOS-0816782/National Science Foundation
- IOS-0816782/National Science Foundation
- IOS-0816782/National Science Foundation
- 2005-35301-15/National Institute of Food and Agriculture
- 2005-35301-15/National Institute of Food and Agriculture
- Institute of Food and Agricultural Sciences Undergraduate Research Internship/University of Florida
LinkOut - more resources
Full Text Sources

