Noncanonical Form of ERAD Regulates Cardiac Hypertrophy
- PMID: 36317534
- PMCID: PMC9797446
- DOI: 10.1161/CIRCULATIONAHA.122.061557
Noncanonical Form of ERAD Regulates Cardiac Hypertrophy
Abstract
Background: Cardiac hypertrophy increases demands on protein folding, which causes an accumulation of misfolded proteins in the endoplasmic reticulum (ER). These misfolded proteins can be removed by the adaptive retrotranslocation, polyubiquitylation, and a proteasome-mediated degradation process, ER-associated degradation (ERAD), which, as a biological process and rate, has not been studied in vivo. To investigate a role for ERAD in a pathophysiological model, we examined the function of the functional initiator of ERAD, valosin-containing protein-interacting membrane protein (VIMP), positing that VIMP would be adaptive in pathological cardiac hypertrophy in mice.
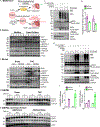
Methods: We developed a new method involving cardiac myocyte-specific adeno-associated virus serovar 9-mediated expression of the canonical ERAD substrate, TCRα, to measure the rate of ERAD, ie, ERAD flux, in the heart in vivo. Adeno-associated virus serovar 9 was also used to either knock down or overexpress VIMP in the heart. Then mice were subjected to transverse aortic constriction to induce pressure overload-induced cardiac hypertrophy.
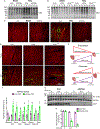
Results: ERAD flux was slowed in both human heart failure and mice after transverse aortic constriction. Surprisingly, although VIMP adaptively contributes to ERAD in model cell lines, in the heart, VIMP knockdown increased ERAD and ameliorated transverse aortic constriction-induced cardiac hypertrophy. Coordinately, VIMP overexpression exacerbated cardiac hypertrophy, which was dependent on VIMP engaging in ERAD. Mechanistically, we found that the cytosolic protein kinase SGK1 (serum/glucocorticoid regulated kinase 1) is a major driver of pathological cardiac hypertrophy in mice subjected to transverse aortic constriction, and that VIMP knockdown decreased the levels of SGK1, which subsequently decreased cardiac pathology. We went on to show that although it is not an ER protein, and resides outside of the ER, SGK1 is degraded by ERAD in a noncanonical process we call ERAD-Out. Despite never having been in the ER, SGK1 is recognized as an ERAD substrate by the ERAD component DERLIN1, and uniquely in cardiac myocytes, VIMP displaces DERLIN1 from initiating ERAD, which decreased SGK1 degradation and promoted cardiac hypertrophy.
Conclusions: ERAD-Out is a new preferentially favored noncanonical form of ERAD that mediates the degradation of SGK1 in cardiac myocytes, and in so doing is therefore an important determinant of how the heart responds to pathological stimuli, such as pressure overload.
Keywords: ER associated protein degradation; SGK1; VIMP; cardiac hypertrophy.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

