Safety and Efficacy of a Monoclonal Antibody against Malaria in Mali
- PMID: 36317783
- PMCID: PMC9881676
- DOI: 10.1056/NEJMoa2206966
Safety and Efficacy of a Monoclonal Antibody against Malaria in Mali
Abstract
Background: CIS43LS is a monoclonal antibody that was shown to protect against controlled Plasmodium falciparum infection in a phase 1 clinical trial. Whether a monoclonal antibody can prevent P. falciparum infection in a region in which the infection is endemic is unknown.
Methods: We conducted a phase 2 trial to assess the safety and efficacy of a single intravenous infusion of CIS43LS against P. falciparum infection in healthy adults in Mali over a 6-month malaria season. In Part A, safety was assessed at three escalating dose levels. In Part B, participants were randomly assigned (in a 1:1:1 ratio) to receive 10 mg of CIS43LS per kilogram of body weight, 40 mg of CIS43LS per kilogram, or placebo. The primary efficacy end point, assessed in a time-to-event analysis, was the first P. falciparum infection detected on blood-smear examination, which was performed at least every 2 weeks for 24 weeks. At enrollment, all the participants received artemether-lumefantrine to clear possible P. falciparum infection.
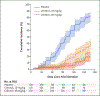
Results: In Part B, 330 adults underwent randomization; 110 were assigned to each trial group. The risk of moderate headache was 3.3 times as high with 40 mg of CIS43LS per kilogram as with placebo. P. falciparum infections were detected on blood-smear examination in 39 participants (35.5%) who received 10 mg of CIS43LS per kilogram, 20 (18.2%) who received 40 mg of CIS43LS per kilogram, and 86 (78.2%) who received placebo. At 6 months, the efficacy of 40 mg of CIS43LS per kilogram as compared with placebo was 88.2% (adjusted 95% confidence interval [CI], 79.3 to 93.3; P<0.001), and the efficacy of 10 mg of CIS43LS per kilogram as compared with placebo was 75.0% (adjusted 95% CI, 61.0 to 84.0; P<0.001).
Conclusions: CIS43LS was protective against P. falciparum infection over a 6-month malaria season in Mali without evident safety concerns. (Funded by the National Institute of Allergy and Infectious Diseases; ClinicalTrials.gov number, NCT04329104.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Monoclonal Antibodies against Malaria.N Engl J Med. 2022 Nov 17;387(20):1898-1899. doi: 10.1056/NEJMe2213148. Epub 2022 Oct 31. N Engl J Med. 2022. PMID: 36317785 No abstract available.
References
-
- World malaria report 2021. Geneva: World Health Organization, 2021:322 (https://www.who.int/teams/global-malaria-programme/reports/world-malaria...).
-
- WHO guidelines for malaria. Geneva: World Health Organization, June 3, 2022 (https://www.who.int/publications/i/item/guidelines-for-malaria).
-
- Ranson H, Lissenden N. Insecticide resistance in African anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol 2016;32:187–96. - PubMed
-
- Balikagala B, Fukuda N, Ikeda M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 2021;385: 1163–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
