Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides
- PMID: 36317800
- PMCID: PMC9744652
- DOI: 10.1152/ajpcell.00147.2022
Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides
Abstract
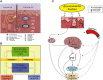
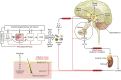
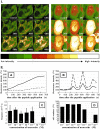
The skin, which is comprised of the epidermis, dermis, and subcutaneous tissue, is the largest organ in the human body and it plays a crucial role in the regulation of the body's homeostasis. These functions are regulated by local neuroendocrine and immune systems with a plethora of signaling molecules produced by resident and immune cells. In addition, neurotransmitters, endocrine factors, neuropeptides, and cytokines released from nerve endings play a central role in the skin's responses to stress. These molecules act on the corresponding receptors in an intra-, juxta-, para-, or autocrine fashion. The epidermis as the outer most component of skin forms a barrier directly protecting against environmental stressors. This protection is assured by an intrinsic keratinocyte differentiation program, pigmentary system, and local nervous, immune, endocrine, and microbiome elements. These constituents communicate cross-functionally among themselves and with corresponding systems in the dermis and hypodermis to secure the basic epidermal functions to maintain local (skin) and global (systemic) homeostasis. The neurohormonal mediators and cytokines used in these communications regulate physiological skin functions separately or in concert. Disturbances in the functions in these systems lead to cutaneous pathology that includes inflammatory (i.e., psoriasis, allergic, or atopic dermatitis, etc.) and keratinocytic hyperproliferative disorders (i.e., seborrheic and solar keratoses), dysfunction of adnexal structure (i.e., hair follicles, eccrine, and sebaceous glands), hypersensitivity reactions, pigmentary disorders (vitiligo, melasma, and hypo- or hyperpigmentary responses), premature aging, and malignancies (melanoma and nonmelanoma skin cancers). These cellular, molecular, and neural components preserve skin integrity and protect against skin pathologies and can act as "messengers of the skin" to the central organs, all to preserve organismal survival.
Keywords: epidermis; keratinocytes; melanocytes; neurohormones; neuropeptides.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR071189/AR/NIAMS NIH HHS/United States
- R21 AR066505/AR/NIAMS NIH HHS/United States
- R21 ES034595/ES/NIEHS NIH HHS/United States
- U54 TR001005/TR/NCATS NIH HHS/United States
- U01 CA223976/CA/NCI NIH HHS/United States
- R01 AR073004/AR/NIAMS NIH HHS/United States
- R01 CA193885/CA/NCI NIH HHS/United States
- I01 BX004293/BX/BLRD VA/United States
- R01 AR047079/AR/NIAMS NIH HHS/United States
- R01 AR056666/AR/NIAMS NIH HHS/United States
- R01 AR052190/AR/NIAMS NIH HHS/United States
- R21 AI149267/AI/NIAID NIH HHS/United States
- U01 AR078544/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

