MRI Quantification of Placebo Effect in Nonalcoholic Steatohepatitis Clinical Trials
- PMID: 36318027
- PMCID: PMC9968769
- DOI: 10.1148/radiol.220743
MRI Quantification of Placebo Effect in Nonalcoholic Steatohepatitis Clinical Trials
Abstract
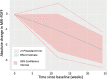
Background Several early-phase clinical trials for the treatment of nonalcoholic steatohepatitis (NASH) use liver fat content as measured with the MRI-derived proton density fat fraction (PDFF) for a primary outcome. These trials have shown relative reductions in liver fat content with placebo treatment alone, a phenomenon termed "the placebo effect." This phenomenon confounds the results and limits generalizability to future trials. Purpose To quantify the effect of placebo treatment on change in the absolute PDFF value and to identify variables associated with this observed change. Materials and Methods This is a secondary analysis of prospectively collected data from seven early phase clinical trials that included participants with a diagnosis of NASH based on MRI and/or liver biopsy who received placebo treatment. The primary outcome was a greater than or equal to 30% relative reduction in PDFF after placebo treatment. Normalization of PDFF, relative change in alanine aminotransferase (ALT) level, and normalization of ALT level were also examined. An exploratory linear mixed-effects model was used to estimate an overall change in absolute PDFF and to explore parameters associated with this response. Results A total of 187 participants (median age, 52 years [IQR, 43-60 years]; 114 women) who received placebo treatment were evaluated. A greater than or equal to 30% relative reduction in baseline PDFF was seen in 20% of participants after 12 weeks of placebo treatment (10 of 49), 9% of participants after 16 weeks (two of 22), and 28% of participants after 24 weeks (34 of 122). A repeated-measures linear mixed-effects model estimated a decrease of 2.3 units (median relative reduction of 13%) in absolute PDFF values after 24 weeks of placebo treatment (95% CI: 3.2, 1.4; P < .001). Conclusion In this analysis of 187 participants, a clinically relevant decrease in PDFF was observed with placebo treatment. Based on the study model, assuming an absolute PDFF decrease of approximately 3 units (upper limit of 95% CI) to account for this "placebo effect" in sample size calculations for future clinical trials is suggested. Clinical trial registration nos. NCT01066364, NCT01766713, NCT01963845, NCT02443116, NCT02546609, NCT02316717, and NCT02442687 © RSNA, 2022 Online supplemental material is available for this article. See also the editorial by Yoon in this issue.
Conflict of interest statement
Figures


Comment in
-
A Placebo Effect on MRI Proton Density Fat Fraction in Nonalcoholic Steatohepatitis Trials.Radiology. 2023 Mar;306(3):e222295. doi: 10.1148/radiol.222295. Epub 2022 Nov 1. Radiology. 2023. PMID: 36318034 No abstract available.
References
-
- Vernon G , Baranova A , Younossi ZM . Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults . Aliment Pharmacol Ther 2011. ; 34 ( 3 ): 274 – 285 . - PubMed
-
- LaBrecque DR , Abbas Z , Anania F , et al. . World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis . J Clin Gastroenterol 2014. ; 48 ( 6 ): 467 – 473 . - PubMed
-
- Williams CD , Stengel J , Asike MI , et al. . Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study . Gastroenterology 2011. ; 140 ( 1 ): 124 – 131 . - PubMed
-
- Chalasani N , Younossi Z , Lavine JE , et al. . The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association . Hepatology 2012. ; 55 ( 6 ): 2005 – 2023 . - PubMed
-
- Mashhood A , Railkar R , Yokoo T , et al. . Reproducibility of hepatic fat fraction measurement by magnetic resonance imaging . J Magn Reson Imaging 2013. ; 37 ( 6 ): 1359 – 1370 . - PubMed

