Multiphase coalescence mediates Hippo pathway activation
- PMID: 36318920
- PMCID: PMC9669202
- DOI: 10.1016/j.cell.2022.09.036
Multiphase coalescence mediates Hippo pathway activation
Abstract
The function of biomolecular condensates is often restricted by condensate dissolution. Whether condensates can be suppressed without condensate dissolution is unclear. Here, we show that upstream regulators of the Hippo signaling pathway form functionally antagonizing condensates, and their coalescence into a common phase provides a mode of counteracting the function of biomolecular condensates without condensate dissolution. Specifically, the negative regulator SLMAP forms Hippo-inactivating condensates to facilitate pathway inhibition by the STRIPAK complex. In response to cell-cell contact or osmotic stress, the positive regulators AMOT and KIBRA form Hippo-activating condensates to facilitate pathway activation. The functionally antagonizing SLMAP and AMOT/KIBRA condensates further coalesce into a common phase to inhibit STRIPAK function. These findings provide a paradigm for restricting the activity of biomolecular condensates without condensate dissolution, shed light on the molecular principles of multiphase organization, and offer a conceptual framework for understanding upstream regulation of the Hippo signaling pathway.
Keywords: AMOT; Hippo pathway; KIBRA; STRIPAK; YAP; biomolecular condensates; cell-cell contact; cytoskeleton; multiphase; osmotic stress.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
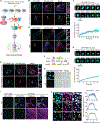
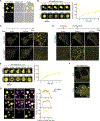
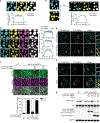


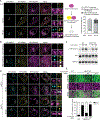

Similar articles
-
Targeted perturbation of signaling-driven condensates.Mol Cell. 2023 Nov 16;83(22):4141-4157.e11. doi: 10.1016/j.molcel.2023.10.023. Epub 2023 Nov 16. Mol Cell. 2023. PMID: 37977121
-
STK25 suppresses Hippo signaling by regulating SAV1-STRIPAK antagonism.Elife. 2020 Apr 15;9:e54863. doi: 10.7554/eLife.54863. Elife. 2020. PMID: 32292165 Free PMC article.
-
Phase separation of Hippo signalling complexes.EMBO J. 2023 Mar 15;42(6):e112863. doi: 10.15252/embj.2022112863. Epub 2023 Feb 20. EMBO J. 2023. PMID: 36807601 Free PMC article.
-
KIBRA connects Hippo signaling and cancer.Exp Cell Res. 2021 Jun 15;403(2):112613. doi: 10.1016/j.yexcr.2021.112613. Epub 2021 Apr 24. Exp Cell Res. 2021. PMID: 33901448 Review.
-
Hippo Signaling in Embryogenesis and Development.Trends Biochem Sci. 2021 Jan;46(1):51-63. doi: 10.1016/j.tibs.2020.08.008. Epub 2020 Sep 11. Trends Biochem Sci. 2021. PMID: 32928629 Free PMC article. Review.
Cited by
-
Atypical Hippo signaling network: uncovering novel insights into head and neck cancer biology and advancements in precision intervention.Front Cell Dev Biol. 2025 May 23;13:1610471. doi: 10.3389/fcell.2025.1610471. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40486910 Free PMC article. Review.
-
LATS2 condensates organize signalosomes for Hippo pathway signal transduction.Nat Chem Biol. 2024 Jun;20(6):710-720. doi: 10.1038/s41589-023-01516-x. Epub 2024 Jan 10. Nat Chem Biol. 2024. PMID: 38200110
-
Emerging regulatory mechanisms and functions of biomolecular condensates: implications for therapeutic targets.Signal Transduct Target Ther. 2025 Jan 6;10(1):4. doi: 10.1038/s41392-024-02070-1. Signal Transduct Target Ther. 2025. PMID: 39757214 Free PMC article. Review.
-
Complex interplay between RAS GTPases and RASSF effectors regulates subcellular localization of YAP.EMBO Rep. 2024 Aug;25(8):3574-3600. doi: 10.1038/s44319-024-00203-9. Epub 2024 Jul 15. EMBO Rep. 2024. PMID: 39009833 Free PMC article.
-
Liquid-Liquid Phase Separation Sheds New Light upon Cardiovascular Diseases.Int J Mol Sci. 2023 Oct 21;24(20):15418. doi: 10.3390/ijms242015418. Int J Mol Sci. 2023. PMID: 37895097 Free PMC article. Review.
References
-
- Adler JJ, Johnson DE, Heller BL, Bringman LR, Ranahan WP, Conwell MD, Sun Y, Hudmon A, and Wells CD (2013). Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proceedings of the National Academy of Sciences of the United States of America 110, 17368–17373. - PMC - PubMed
-
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, and Piccolo S (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

