Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer
- PMID: 36320056
- PMCID: PMC9623991
- DOI: 10.1186/s12943-022-01671-0
Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer
Abstract
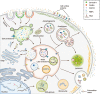
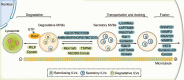
Exosomes are well-known key mediators of intercellular communication and contribute to various physiological and pathological processes. Their biogenesis involves four key steps, including cargo sorting, MVB formation and maturation, transport of MVBs, and MVB fusion with the plasma membrane. Each process is modulated through the competition or coordination of multiple mechanisms, whereby diverse repertoires of molecular cargos are sorted into distinct subpopulations of exosomes, resulting in the high heterogeneity of exosomes. Intriguingly, cancer cells exploit various strategies, such as aberrant gene expression, posttranslational modifications, and altered signaling pathways, to regulate the biogenesis, composition, and eventually functions of exosomes to promote cancer progression. Therefore, exosome biogenesis-targeted therapy is being actively explored. In this review, we systematically summarize recent progress in understanding the machinery of exosome biogenesis and how it is regulated in the context of cancer. In particular, we highlight pharmacological targeting of exosome biogenesis as a promising cancer therapeutic strategy.
Keywords: Biogenesis; Cancer; Exosomes; Implications; Regulation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

