3-(4-Formylphenyl)-triazole functionalized coumarins as violet-blue luminophores and n-type semiconductors: synthesis, photophysical, electrochemical and thermal properties
- PMID: 36320249
- PMCID: PMC9527578
- DOI: 10.1039/d2ra03266j
3-(4-Formylphenyl)-triazole functionalized coumarins as violet-blue luminophores and n-type semiconductors: synthesis, photophysical, electrochemical and thermal properties
Abstract
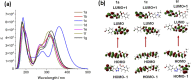
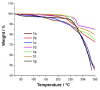
3-(4-Formylphenyl)-triazole-coumarin hybrid chromophores (FPhTCs) were synthesized in good yields, using a click chemistry protocol, and were also structurally characterized. Their photophysical, electrochemical and thermal properties were measured demonstrating that FPhTCs are luminescent in the blue-violet region of the electromagnetic spectrum, both in solution and the solid state. They showed an electrochemical band-gap values of 2.79 ± 0.08 eV, resistivity values between 104 and 105 Ω cm and are thermally stable up to 225 °C, properties that promise FPhTCs as good candidates for optoelectronic or imaging applications. Their solution and solid state photoluminescent properties are discussed and supported by theoretical calculations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Fang B. Li P. Jiang J. Du W. Wang L. Bai H. Peng B. Huang X. An Z. Li L. Yang X. Fu L. Huang W. Coord. Chem. Rev. 2021;440:213979.
-
- Yang Z. Fan X. Li H. Li X. Li S. Zhang Z. Lin H. Qian J. Hua J. Chem.–Eur. J. 2021;27:14240. - PubMed
-
- Han J. Guo S. Lu H. Liu S. Zhao Q. Huang W. Adv. Opt. Mater. 2018;6:1800538.
-
- He G. Organic Optoelectronic Materials. Lect. Notes Chem. 2015;91:241.
LinkOut - more resources
Full Text Sources
Miscellaneous

