Pillar[ n]arene-calix[ m]arene hybrid macrocyclic structures
- PMID: 36320255
- PMCID: PMC9528731
- DOI: 10.1039/d2ra05118d
Pillar[ n]arene-calix[ m]arene hybrid macrocyclic structures
Abstract
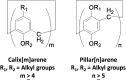
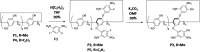
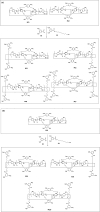
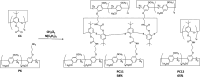
To reserve planar chirality, enhance molecular recognition, and build advanced self-assemblies, hybrid macrocyclic hosts containing rigid pillar[n]arene and flexible calix[m]arene were designed, prepared and investigated for interesting applications. This review summarizes and discusses different synthetic strategies for constructing hybrid macrocyclic structures. Pillar[n]arene dimer with rigid aromatic double bridges provided the possibility of introducing calix[m]arene cavities, where the planar chirality was reserved in the structure of pillararene. The capacity for molecular recognition was enhanced by hybrid macrocyclic cavities. Interestingly, the obtained pillar[n]arene-calix[m]arene could self-assemble into "channels" and "honeycomb" in both the solid state and solution phase as well as donate the molecular architecture as the wheel for the formation of mechanically interlocked molecules, such as rotaxane. In addition, the pillar[n]arene and calix[m]arene could also be coupled together to produce pillar[n]arene embeded 1,3-alternate and cone conformational calix[m]arene derivatives, which could catalyze the oxidative polymerization of aniline in aqueous solutions. Except for building hybrid cyclophanes by covalent bonds, weak supramolecular interactions were used to prepare pillar[n]arene-calix[m]arene analogous composites with other pillar-like pillar[n]pyridiniums and calix-like calix[m]pyrroles, exhibiting reasonable performances in enhancing molecular recognition and trapping solvent molecules.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










Similar articles
-
Fullerene-containing pillar[n]arene hybrid composites.Org Biomol Chem. 2022 Nov 2;20(42):8176-8186. doi: 10.1039/d2ob01664h. Org Biomol Chem. 2022. PMID: 36226561 Review.
-
Cyclodextrin-pillar[n]arene hybridized macrocyclic systems.Org Biomol Chem. 2022 Jun 1;20(21):4278-4288. doi: 10.1039/d2ob00671e. Org Biomol Chem. 2022. PMID: 35552579 Review.
-
Hybrid Macrocyclic Polymers: Self-Assembly Containing Cucurbit[m]uril-pillar[n]arene.Polymers (Basel). 2022 Apr 27;14(9):1777. doi: 10.3390/polym14091777. Polymers (Basel). 2022. PMID: 35566949 Free PMC article. Review.
-
Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[ n]arenes.Acc Chem Res. 2018 Jul 17;51(7):1656-1666. doi: 10.1021/acs.accounts.8b00157. Epub 2018 Jun 11. Acc Chem Res. 2018. PMID: 29889488
-
Calix[n]arene/Pillar[n]arene-Functionalized Graphene Nanocomposites and Their Applications.Front Chem. 2020 Jun 12;8:504. doi: 10.3389/fchem.2020.00504. eCollection 2020. Front Chem. 2020. PMID: 32596211 Free PMC article. Review.
References
-
- Yang G. Wu L. Chen G. Jiang M. Chem. Commun. 2016;52:10595–10605. - PubMed
-
- Sun X. Zhai W. Fossey J. S. James T. D. Chem. Commun. 2016;52:3456–3469. - PubMed
-
- Li W. Bockus A. T. Vinciguerra B. Isaacs L. Urbach A. R. Chem. Commun. 2016;52:8537–8540. - PubMed
-
- Raiha N. C. Somersalo O. Nordstrom C. G. Raiha C. E. Acta Paediatr. 1962;135:171–177. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

