Design, synthesis, and evaluation of chiral thiophosphorus acids as organocatalysts
- PMID: 36320342
- PMCID: PMC9592963
- DOI: 10.3762/bjoc.18.154
Design, synthesis, and evaluation of chiral thiophosphorus acids as organocatalysts
Abstract
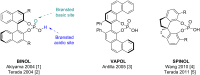
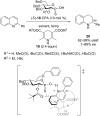
A series of P-stereogenic chiral phosphorus acids (CPAs) were synthesized to determine the requirements for efficient asymmetric organocatalysis. In order to eliminate the need for C 2-symmetry in common CPAs, various scaffolds containing C 1-symmetrical thiophosphorus acids were chosen. These new compounds were synthesized and evaluated in the asymmetric transfer hydrogenation of 2-phenylquinoline. Although the efficacy of the thiophosphorus acids was disappointing for this reaction, the work should be useful for developing structural design elements.
Keywords: asymmetric; heterocycles; organocatalysis; phosphorus; synthesis.
Copyright © 2022, Winters and Montchamp.
Figures






References
-
- Terada M. Curr Org Chem. 2011;15:2227–2256. doi: 10.2174/138527211796150732. - DOI
LinkOut - more resources
Full Text Sources
