Imaging manifestations of von Hippel-Lindau disease: an illustrated guide focusing on abdominal manifestations
- PMID: 36320367
- PMCID: PMC9620840
- DOI: 10.1590/0100-3984.2021.0121-en
Imaging manifestations of von Hippel-Lindau disease: an illustrated guide focusing on abdominal manifestations
Abstract
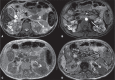
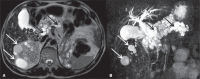
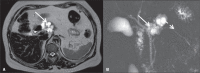
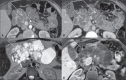
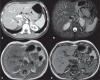
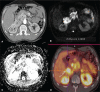
Von Hippel-Lindau (VHL) disease is a monogenic autosomal dominant disorder with germline mutations of the VHL anti-oncogene on the short arm of chromosome 3 (3p25-26). It affects 1:36,000-50,000 individuals, with a penetrance greater than 90% at 65 years of age. Although of variable onset and presentation, with pleiotropism even among members of the same family who share a specific mutation, VHL disease usually manifests initially in young adults. It predisposes to the development of benign and malignant tumors of the central nervous system (CNS) and visceral organs. The clinical diagnosis of VHL disease can be made in the following circumstances: a) in patients with a family history of the disease and at least one of the tumors characteristic of it (e.g., retinal or CNS hemangioblastomas, clear cell renal cell carcinoma, pancreatic neuroendocrine tumors, and endolymphatic sac tumors); b) in patients with two or more CNS hemangioblastomas; c) or in patients with a retinal or CNS hemangioblastoma plus at least one visceral tumor characteristic of the disease, excluding renal and epididymal cysts. Imaging plays an important role in the diagnosis and follow-up of patients with VHL disease. This pictorial essay presents characteristic images of abdominal manifestations of VHL disease-related tumors that all radiologists should be aware of.
A doença de von Hippel-Lindau (VHL) é uma desordem autossômica dominante monogênica com mutações na linha germinativa do antioncogene VHL, no braço curto do cromossomo três (3p25-26). Afeta 1:36.000-50.000 indivíduos, com penetrância superior a 90% aos 65 anos de idade. Embora tenha início e apresentação variáveis, com pleiotropismo mesmo entre membros da mesma família que partilham uma mutação específica, usualmente manifesta-se de início em adultos jovens e predispõe ao desenvolvimento de tumores benignos e malignos no sistema nervoso central (SNC) e órgãos viscerais. Clinicamente, o diagnóstico pode ser realizado em uma das seguintes circunstâncias: a) em pacientes com história familiar de doença de VHL e pelo menos um dos tumores característicos relacionados à síndrome (como hemangioblastomas retinianos ou do SNC, carcinoma de células renais de células claras, tumores neuroendócrinos pancreáticos e tumores do saco endolinfático); b) dois ou mais hemangioblastomas do SNC; c) um hemangioblastoma retiniano ou do SNC mais pelo menos um tumor característico visceral relacionado à síndrome, excluindo-se cistos renais e epididimários. Nesse contexto, a imagem ocupa importante papel no diagnóstico e acompanhamento desses pacientes. Este ensaio iconográfico apresenta imagens características de manifestações abdominais de tumores relacionados à doença de VHL que todos os radiologistas devem conhecer.
Keywords: Carcinoma; Pancreatic neoplasms; Pheochromocytoma; renal cell; von Hippel-Lindau disease/diagnostic imaging.
Figures
References
-
- Ganeshan D, Menias CO, Pickhardt PJ, et al. Tumors in von Hip-pel-Lindau syndrome: from head to toe-comprehensive state-ofthe-art review. Radiographics. 2018;38:849–866. - PubMed
-
- Tappouni R, Kissane J, Sarwani N, et al. Pseudoenhancement of renal cysts: influence of lesion size, lesion location, slice thickness, and number of MDCT detectors. AJR Am J Roentgenol. 2012;198:133–137. - PubMed
-
- Choi YA, Kim CK, Park BK, et al. Evaluation of adrenal metastases from renal cell carcinoma and hepatocellular carcinoma: use of delayed contrast-enhanced CT. Radiology. 2013;266:514–520. - PubMed
LinkOut - more resources
Full Text Sources
