Mechanochemical ring-opening metathesis polymerization: development, scope, and mechano-exclusive polymer synthesis
- PMID: 36320385
- PMCID: PMC9557243
- DOI: 10.1039/d2sc02536a
Mechanochemical ring-opening metathesis polymerization: development, scope, and mechano-exclusive polymer synthesis
Abstract
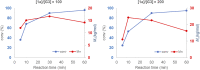
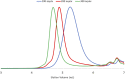
Ruthenium-alkylidene initiated ring-opening metathesis polymerization has been realized under solid-state conditions by employing a mechanochemical ball milling method. This method promotes greenness and broadens the scope to include mechano-exclusive products. The carbene- and pyridine-based Grubbs 3rd-generation complex outperformed other catalysts and maintained similar mechanistic features of solution-phase reactions. High-speed ball milling provides sufficient mixing and energy to the solid reaction mixture, which is composed of an initiator and monomers, to minimize or eliminate the use of solvents. Therefore, the solubility and miscibility of monomers and Ru-initiators are not limiting factors in solid-state ball milling. A wide variety of solid monomers, including ionomers, fluorous monomers, and macromonomers, were successfully polymerized under ball milling conditions. Importantly, direct copolymerization of immiscible (ionic/hydrophobic) monomers exemplifies the synthesis of mechano-exclusive polymers that are difficult to make using traditional solution procedures. Finally, the addition of a small amount of a liquid additive (i.e., liquid-assisted grinding) minimized chain-degradation, enabling high-molecular-weight polymer synthesis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Bielawski C. W. Grubbs R. H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007;32:1–29. doi: 10.1016/j.progpolymsci.2006.08.006. - DOI
- Sutthasupa S. Shiotsuki M. Sanda F. Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym. J. 2010;42:905–915. doi: 10.1038/pj.2010.94. - DOI
- Ogba O. M. Warner N. C. O'Leary D. J. Grubbs R. H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 2018;47:4510–4544. doi: 10.1039/C8CS00027A. - DOI - PMC - PubMed
-
- Skowerski K. Biatecki J. Tracz A. Olszewski T. K. An attempt to provide an environmentally friendly solvent selection guide for olefin metathesis. Green Chem. 2014;16:1125–1130. doi: 10.1039/C3GC41943F. - DOI
- Fürstner A. Ackermann L. Beck K. Hori H. Koch D. Langmann K. Liebl M. Six C. Leitner W. Olefin metathesis in supercritical carbon dioxide. J. Am. Chem. Soc. 2001;123:9000–9006. doi: 10.1021/ja010952k. - DOI - PubMed
- Shin H. G. Lee H. S. Hong E. J. Kim J. G. Study of green solvents for ruthenium alkylidene mediated ring-opening Metathesis Polymerization. Bull. Korean Chem. Soc. 2021;42:502–505. doi: 10.1002/bkcs.12213. - DOI
- Shetty M. Kothapalli V. A. Hobbs C. E. Toward the (nearly) complete elimination of solvent waste in ring opening metathesis polymerization (ROMP) reactions. Polymer. 2015;80:64–66. doi: 10.1016/j.polymer.2015.10.036. - DOI
-
- James S. L. Friščić T. Mechanochemistry. Chem. Soc. Rev. 2013;42:7494–7496. doi: 10.1039/C3CS90058D. - DOI - PubMed
- James S. L. Friščić T. Mechanochemistry: A web themed issue. Chem. Commun. 2013;49:5349–5350. doi: 10.1039/C3CC90136J. - DOI - PubMed
- Gilman J. J. Mechanochemistry. Science. 1996;274:65. doi: 10.1126/science.274.5284.65. - DOI
-
- James S. L. Adams C. J. Bolm C. Braga D. Collier P. Friščić T. Grepioni F. Harris K. D. M. Hyett G. Jones W. Krebs A. Mack J. Maini L. Orpen A. G. Parkin I. P. Shearouse W. C. Steed J. W. Waddell D. C. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012;41:413–447. doi: 10.1039/C1CS15171A. - DOI - PubMed
- Hernández J. G. Mechanochemistry. Beilstein J. Org. Chem. 2017;13:2372–2373. doi: 10.3762/bjoc.13.234. - DOI - PMC - PubMed
- Do J.-L. Friščić T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017;3:13–19. doi: 10.1021/acscentsci.6b00277. - DOI - PMC - PubMed
- Friščić T. Mottillo C. Titi H. M. Mechanochemistry for synthesis. Angew. Chem., Int. Ed. 2020;59:1018–1029. doi: 10.1002/anie.201906755. - DOI - PubMed
- Ardila-Fierro K. J. Hernández J. G. Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem. 2021;14:2145–2162. doi: 10.1002/cssc.202100478. - DOI - PubMed
-
- Wang G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013;42:7668–7700. doi: 10.1039/C3CS35526H. - DOI - PubMed
- Porcheddu A. Colacino E. De Luca L. Delogu F. Metal-mediated and metal-catalyzed reactions under mechanochemical conditions. ACS Catal. 2020;10:8344–8394. doi: 10.1021/acscatal.0c00142. - DOI
- Stolle A. Szuppa T. Leonhardt S. E. S. Ondruschka B. Ball milling in organic synthesis: solutions and challenges. Chem. Soc. Rev. 2011;40:2317–2329. doi: 10.1039/C0CS00195C. - DOI - PubMed
- Tan D. Friščić T. Mechanochemistry for organic chemists: An update. Eur. J. Org. Chem. 2018;2018:18–33. doi: 10.1002/ejoc.201700961. - DOI
- Rightmire N. R. Hanusa T. P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016;45:2352–2362. doi: 10.1039/C5DT03866A. - DOI - PubMed
- Moores A. Bottom up, solid-phase syntheses of inorganic nanomaterials by mechanochemistry and aging. Curr. Opin. Green Sustainable Chem. 2018;12:33–37. doi: 10.1016/j.cogsc.2018.05.004. - DOI
- Boldyreva E. Mechanochemistry of inorganic and organic systems: what is similar, what is different? Chem. Soc. Rev. 2013;42:7719–7738. doi: 10.1039/C3CS60052A. - DOI - PubMed
- Tan D. García F. Main group mechanochemistry: from curiosity to established protocols. Chem. Soc. Rev. 2019;48:2274–2292. doi: 10.1039/C7CS00813A. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

