Fluorine in metal-catalyzed asymmetric transformations: the lightest halogen causing a massive effect
- PMID: 36320478
- PMCID: PMC9516955
- DOI: 10.1039/d2sc01096h
Fluorine in metal-catalyzed asymmetric transformations: the lightest halogen causing a massive effect
Abstract
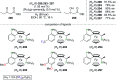
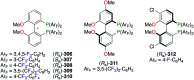
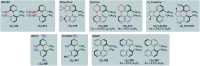
This review aims at providing an overview of the most significant applications of fluorine-containing ligands reported in the literature starting from 2001 until mid-2021. The ligands are classified according to the nature of the donor atoms involved. This review highlights both metal-ligand interactions and the structure-reactivity relationships resulting from the presence of the fluorine atom or fluorine-containing substituents on chiral catalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



















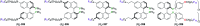


Similar articles
-
Further Developments and Applications of Oxazoline-Containing Ligands in Asymmetric Catalysis.Chem Rev. 2021 Jun 9;121(11):6373-6521. doi: 10.1021/acs.chemrev.0c00844. Epub 2021 May 21. Chem Rev. 2021. PMID: 34019404 Free PMC article.
-
Iron- and Cobalt-Catalyzed Asymmetric Hydrofunctionalization of Alkenes and Alkynes.Acc Chem Res. 2021 Jun 1;54(11):2701-2716. doi: 10.1021/acs.accounts.1c00212. Epub 2021 May 19. Acc Chem Res. 2021. PMID: 34011145
-
Highly Efficient Asymmetric Hydrogenation Catalyzed by Iridium Complexes with Tridentate Chiral Spiro Aminophosphine Ligands.Acc Chem Res. 2023 Feb 7;56(3):332-349. doi: 10.1021/acs.accounts.2c00764. Epub 2023 Jan 23. Acc Chem Res. 2023. PMID: 36689780
-
Chiral Diene Ligands in Asymmetric Catalysis.Chem Rev. 2022 Sep 28;122(18):14346-14404. doi: 10.1021/acs.chemrev.2c00218. Epub 2022 Aug 16. Chem Rev. 2022. PMID: 35972018 Review.
-
Transition-Metal-Mediated and -Catalyzed C-F Bond Activation by Fluorine Elimination.Angew Chem Int Ed Engl. 2019 Jan 8;58(2):390-402. doi: 10.1002/anie.201805292. Epub 2018 Nov 8. Angew Chem Int Ed Engl. 2019. PMID: 29953707 Review.
Cited by
-
A Dual Role for the N-Perfluorobutanesulfinamide Auxiliary in an Asymmetric Decarboxylative Mannich Reaction.Org Lett. 2024 Oct 18;26(41):8810-8815. doi: 10.1021/acs.orglett.4c03139. Epub 2024 Sep 30. Org Lett. 2024. PMID: 39348273 Free PMC article.
References
-
- Seppelt K., The Curious World of Fluorinated Molecules: Molecules Containing Fluorine, Elsevier, Amsterdam, 2021
- Tressaud A., Fluorine: A Paradoxal Element, Elsevier, Amsterdam, 2019
- Kirsch P., Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Weinheim, 2013
-
- O'Hagan D. Harper D. B. J. Fluorine Chem. 1999;100:127–133. doi: 10.1016/S0022-1139(99)00201-8. - DOI
-
- Mei H. Han J. Fustero S. Medio-Simon M. Sedgwick D. M. Santi C. Ruzziconi R. Soloshonok V. A. Chem.–Eur. J. 2019;25:11797–11819. doi: 10.1002/chem.201901840. - DOI - PubMed
- Zhou Y. Wang J. Gu Z. Wang S. Zhu W. Aceña J. L. Soloshonok V. A. Izawa K. Liu H. Chem. Rev. 2016;116:422–518. doi: 10.1021/acs.chemrev.5b00392. - DOI - PubMed
- Wang J. Sánchez-Roselló M. Aceña J. L. del Pozo C. Sorochinsky A. E. Fustero S. Soloshonok V. A. Liu H. Chem. Rev. 2014;114:2432–2506. doi: 10.1021/cr4002879. - DOI - PubMed
-
- Meanwell N. A. J. Med. Chem. 2018;61:5822–5880. doi: 10.1021/acs.jmedchem.7b01788. - DOI - PubMed
- Gillis E. P. Eastman K. J. Hill M. D. Donnelly D. J. Meanwell N. A. J. Med. Chem. 2015;58:8315–8359. doi: 10.1021/acs.jmedchem.5b00258. - DOI - PubMed
- Hagmann W. K. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. - DOI - PubMed
- Purser S. Moore P. R. Swallow S. Gouverneur V. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. - DOI - PubMed
- Müller K. Faeh C. Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

