A dinickel-catalyzed three-component cycloaddition of vinylidenes
- PMID: 36320482
- PMCID: PMC9516891
- DOI: 10.1039/d2sc02696a
A dinickel-catalyzed three-component cycloaddition of vinylidenes
Abstract
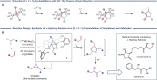
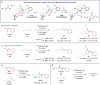
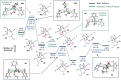
A dinickel catalyst promotes the [2 + 2 + 1]-cycloaddition of two aldehyde equivalents and a vinylidene. The resulting methylenedioxolane products can be deprotected in one pot under acidic conditions to reveal α-hydroxy ketones. This method provides convenient access to unsymmetrical alkyl-substituted α-hydroxy ketones, which are challenging to synthesize selectively using cross-benzoin reactions. Mechanistic studies are consistent with an initial migratory insertion of the aldehyde into a dinickel bridging vinylidene. Insertion of the second aldehyde followed by C-O reductive elimination furnishes the cycloadduct. Under dilute conditions, an enone side product is generated due to a competing β-hydride elimination from the proposed metallacyclic intermediate. A DFT model consistent with the concentration-dependent formation of the methylenedioxolane and enone is presented.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Pauson P. L. Tetrahedron. 1985;41:5855–5860.
- Brummond K. M. Kent J. L. Tetrahedron. 2000;56:3263–3283.
- Schore N. E. Org. React. 1991;40:1–90.
-
- Hoshimoto Y. Ohata T. Sasaoka Y. Ohashi M. Ogoshi S. J. Am. Chem. Soc. 2014;136:15877–15880. - PubMed
-
- Kablaoui N. M. Hicks F. A. Buchwald S. L. J. Am. Chem. Soc. 1996;118:5818–5819.
- Chatani N. Morimoto T. Fukumoto Y. Murai S. J. Am. Chem. Soc. 1998;120:5335–5336.
- Adrio J. Carretero J. C. J. Am. Chem. Soc. 2007;129:778–779. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

