A switchable redox annulation of 2-nitroarylethanols affording N-heterocycles: photoexcited nitro as a multifunctional handle
- PMID: 36320483
- PMCID: PMC9516892
- DOI: 10.1039/d2sc03590a
A switchable redox annulation of 2-nitroarylethanols affording N-heterocycles: photoexcited nitro as a multifunctional handle
Abstract
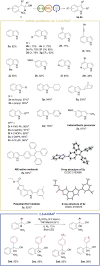
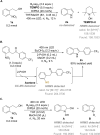
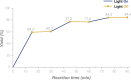
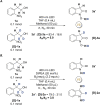
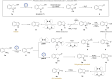
The efficient transformation of nitroaromatics to functional molecules such as N-heterocycles has been an attractive and significant topic in synthesis chemistry. Herein, a photoexcited nitro-induced strategy for switchable annulations of 2-nitroarylethanols was developed to construct N-heterocycles including indoles, N-hydroxyl oxindoles and N-H oxindoles. The metal- and photocatalyst-free reaction proceeds through intramolecular redox C-N coupling of branched hydroxyalkyl and nitro units, which is initiated by a double hydrogen atom abstraction (d-HAA) process. The key to the switchable reaction outcomes is the mediation of a diboron reagent by its favorable oxy-transfer reactivity to in situ generated nitroso species. The utility of this protocol was well demonstrated by broad substrate scope, excellent yields, functional group tolerance and wide applications. Finally, detailed mechanistic studies were performed, and kinetic isotope effect (KIE) experiments indicate that the homolysis of the C-H bond is involved in the rate-determining step.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Road Map for the Construction of High-Valued N-Heterocycles via Denitrogenative Annulation.Acc Chem Res. 2021 Dec 7;54(23):4395-4409. doi: 10.1021/acs.accounts.1c00563. Epub 2021 Nov 11. Acc Chem Res. 2021. PMID: 34761918
-
Catalytic reaction profile for alcohol oxidation by galactose oxidase.Biochemistry. 2001 Jun 19;40(24):7140-8. doi: 10.1021/bi010303l. Biochemistry. 2001. PMID: 11401560
-
Transition-metal-free Chemoselective Oxidative C-C Coupling of the sp3 C-H Bond of Oxindoles with Arenes and Addition to Alkene: Synthesis of 3-Aryl Oxindoles, and Benzofuro- and Indoloindoles.Chem Asian J. 2017 Apr 4;12(7):734-743. doi: 10.1002/asia.201601647. Epub 2017 Mar 2. Chem Asian J. 2017. PMID: 28169505
-
Davis-Beirut Reaction: Diverse Chemistries of Highly Reactive Nitroso Intermediates in Heterocycle Synthesis.Acc Chem Res. 2019 Aug 20;52(8):2256-2265. doi: 10.1021/acs.accounts.9b00220. Epub 2019 Jul 22. Acc Chem Res. 2019. PMID: 31328502 Free PMC article. Review.
-
Metal-Free Oxidative B-N Coupling of nido-Carborane with N-Heterocycles.Angew Chem Int Ed Engl. 2019 Aug 19;58(34):11886-11892. doi: 10.1002/anie.201904940. Epub 2019 Jul 9. Angew Chem Int Ed Engl. 2019. PMID: 31233261 Review.
Cited by
-
Merging Photoexcited Nitroarenes with Lewis Acid Catalysis for the Anti-Markovnikov Oxidation of Alkenes.Org Lett. 2025 Feb 28;27(8):2011-2015. doi: 10.1021/acs.orglett.5c00389. Epub 2025 Feb 20. Org Lett. 2025. PMID: 39973366 Free PMC article.
-
Photoexcited nitroarene-enabled carbon chain-elongated oxidation of alkenes via tandem oxidative cleavage and dipolar cycloaddition.Nat Commun. 2025 May 15;16(1):4504. doi: 10.1038/s41467-025-59274-4. Nat Commun. 2025. PMID: 40374615 Free PMC article.
-
Continuous Flow Synthesis of Nitrosoarenes via Photochemical Rearrangement of Aryl Imines.J Org Chem. 2024 Jan 5;89(1):617-623. doi: 10.1021/acs.joc.3c02362. Epub 2023 Dec 22. J Org Chem. 2024. PMID: 38131303 Free PMC article.
-
Anaerobic Hydroxylation of C(sp3)-H Bonds Enabled by the Synergistic Nature of Photoexcited Nitroarenes.J Am Chem Soc. 2023 Feb 8;145(5):2794-2799. doi: 10.1021/jacs.2c13502. Epub 2023 Jan 25. J Am Chem Soc. 2023. PMID: 36696364 Free PMC article.
-
Photoinduced Nitroarenes as Versatile Anaerobic Oxidants for Accessing Carbonyl and Imine Derivatives.Org Lett. 2023 Sep 8;25(35):6517-6521. doi: 10.1021/acs.orglett.3c02292. Epub 2023 Aug 24. Org Lett. 2023. PMID: 37680131 Free PMC article.
References
-
- Janosik T. Rannug A. Rannug U. Wahlström N. Slätt J. Bergman J. Chem. Rev. 2018;118:9058–9128. - PubMed
- Cockerill G. S. Angell R. M. Bedernjak A. Chuckowree I. Fraser I. Gascon-Simorte J. Gilman M. S. A. Good J. A. D. Harland R. Johnson S. M. Ludes-Meyers J. H. Littler E. Lumley J. Lunn G. Mathews N. McLellan J. S. Paradowski M. Peeples M. E. Scott C. Tait D. Taylor G. Thom M. Thomas E. Villalonga Barber C. Ward S. E. Watterson D. Williams G. Young P. Powell K. J. Med. Chem. 2021;64:3658–3676. - PubMed
-
- Sun A. W. Lackner S. Stoltz B. M. Trends Chem. 2019;1:630–643.
LinkOut - more resources
Full Text Sources

