Chronoampermetric detection of enzymatic glucose sensor based on doped polyindole/MWCNT composites modified onto screen-printed carbon electrode as portable sensing device for diabetes
- PMID: 36320500
- PMCID: PMC9535471
- DOI: 10.1039/d2ra04947c
Chronoampermetric detection of enzymatic glucose sensor based on doped polyindole/MWCNT composites modified onto screen-printed carbon electrode as portable sensing device for diabetes
Abstract
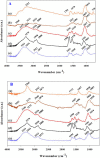
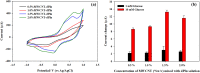
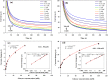
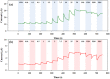
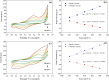
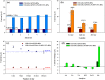
Doped-polyindole (dPIn) mixed with multi-walled carbon nanotubes (MWCNTs) were coated on a screen-printed electrode to improve the electroactive surface area and current response of the chronoamperometric enzymatic glucose sensor. Glucose oxidase mixed with chitosan (CHI-GOx) was immobilized on the electrode. (3-Aminopropyl) triethoxysilane (APTES) was used as a linker between the CHI-GOx and the dPIn. The current response of the glucose sensor increased with increasing glucose concentration according to a power law relation. The sensitivity of the CHI-GOx/APTES/dPIn was 55.7 μA mM-1 cm-2 with an LOD (limit of detection) of 0.01 mM, where the detectable glucose concentration range was 0.01-50 mM. The sensitivity of the CHI-GOx/APTES/1.5%MWCNT-dPIn was 182.9 μA mM-1 cm-2 with an LOD of 0.01 mM, where the detectable glucose concentration range was 0.01-100 mM. The detectable concentration ranges of glucose well cover the glucose concentrations in urine and blood. The fabricated enzymatic glucose sensors showed high stability during a storage period of four weeks and high selectivity relative to other interferences. Moreover, the sensor was successfully demonstrated as a continuous or step-wise glucose monitoring device. The preparation method employed here was facile and suitable for large quantity production. The glucose sensor fabricated here, consisting of the three-electrode cell of SPCE, were simple to use for glucose detection. Thus, it is promising to use as a prototype for real glucose monitoring for diabetic patients in the future.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


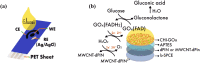
Similar articles
-
Screen-printed electrode designed with MXene/doped-polyindole and MWCNT/doped-polyindole for chronoamperometric enzymatic glucose sensor.Heliyon. 2024 Jan 12;10(2):e24346. doi: 10.1016/j.heliyon.2024.e24346. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293452 Free PMC article.
-
Noninvasive glucose monitoring using portable GOx-Based biosensing system.Anal Chim Acta. 2024 Jan 25;1287:342068. doi: 10.1016/j.aca.2023.342068. Epub 2023 Nov 27. Anal Chim Acta. 2024. PMID: 38182375
-
Enzyme entrapment by β-cyclodextrin electropolymerization onto a carbon nanotubes-modified screen-printed electrode.Biosens Bioelectron. 2010 Dec 15;26(4):1768-73. doi: 10.1016/j.bios.2010.08.058. Epub 2010 Aug 26. Biosens Bioelectron. 2010. PMID: 20863684
-
Glucose oxidase immobilized amine terminated multiwall carbon nanotubes/reduced graphene oxide/polyaniline/gold nanoparticles modified screen-printed carbon electrode for highly sensitive amperometric glucose detection.Mater Sci Eng C Mater Biol Appl. 2019 Dec;105:110075. doi: 10.1016/j.msec.2019.110075. Epub 2019 Aug 12. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31546385
-
Green Fabrication of Nonenzymatic Glucose Sensor Using Multi-Walled Carbon Nanotubes Decorated with Copper (II) Oxide Nanoparticles for Tear Fluid Analysis.Appl Biochem Biotechnol. 2022 Aug;194(8):3689-3705. doi: 10.1007/s12010-022-03936-2. Epub 2022 Apr 30. Appl Biochem Biotechnol. 2022. PMID: 35488956
Cited by
-
Nature-Inspired Biomolecular Corona Based on Poly(caffeic acid) as a Low Potential and Time-Stable Glucose Biosensor.Molecules. 2023 Oct 26;28(21):7281. doi: 10.3390/molecules28217281. Molecules. 2023. PMID: 37959700 Free PMC article.
-
Screen-printed electrode designed with MXene/doped-polyindole and MWCNT/doped-polyindole for chronoamperometric enzymatic glucose sensor.Heliyon. 2024 Jan 12;10(2):e24346. doi: 10.1016/j.heliyon.2024.e24346. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293452 Free PMC article.
-
Fe3O4@Au Core-Shell Magnetic Nanoparticles for the Rapid Analysis of E. coli O157:H7 in an Electrochemical Immunoassay.Biosensors (Basel). 2023 May 22;13(5):567. doi: 10.3390/bios13050567. Biosensors (Basel). 2023. PMID: 37232928 Free PMC article.
-
Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells.Polymers (Basel). 2023 Sep 15;15(18):3783. doi: 10.3390/polym15183783. Polymers (Basel). 2023. PMID: 37765637 Free PMC article. Review.
-
Schottky Interface Enabled Electrospun Rhodium Oxide Doped Gold for Both pH Sensing and Glucose Measurements in Neutral Buffer and Human Serum.Langmuir. 2024 Oct 1;40(39):20797-20810. doi: 10.1021/acs.langmuir.4c02999. Epub 2024 Sep 17. Langmuir. 2024. PMID: 39287604 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

