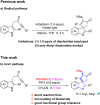
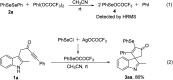
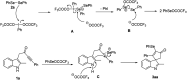
PIFA-mediated selenylative spirocyclization of indolyl ynones: facile access to selenated spiro[cyclopentenone-1,3'-indoles]
- PMID: 36320507
- PMCID: PMC9549584
- DOI: 10.1039/d2ra05387j
PIFA-mediated selenylative spirocyclization of indolyl ynones: facile access to selenated spiro[cyclopentenone-1,3'-indoles]
Abstract
A fast selenylative spirocyclization of indolyl ynones mediated by PIFA has been developed. This transformation was enabled by the reactive RSeOCOCF3 species generated in situ from diselenides with PIFA, involving an electrophilic dearomative cascade cyclization. This protocol provides a facile and efficient method for the synthesis of selenated spiro[cyclopentenone-1,3'-indoles] and tolerates broad functional groups.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Visible light-induced selenylative spirocyclization of biaryl ynones toward the formation of selenated spiro[5.5]trienones.Org Biomol Chem. 2022 Jul 27;20(29):5779-5783. doi: 10.1039/d2ob01006b. Org Biomol Chem. 2022. PMID: 35815996
-
Metal-Free Sulfonylative Spirocyclization of Indolyl-ynones via Insertion of Sulfur Dioxide: Access to Sulfonated Spiro[cyclopentenone-1,3'-indoles].Org Lett. 2021 Oct 15;23(20):7992-7995. doi: 10.1021/acs.orglett.1c02999. Epub 2021 Sep 28. Org Lett. 2021. PMID: 34581591
-
Visible-Light-Promoted Selenylative Spirocyclization of Indolyl-ynones toward the Formation of 3-Selenospiroindolenine Anticancer Agents.Chem Asian J. 2020 May 15;15(10):1536-1539. doi: 10.1002/asia.202000298. Epub 2020 Apr 7. Chem Asian J. 2020. PMID: 32207240
-
BBr3-mediated dearomative spirocyclization of biaryl ynones: facile access to spiro[5.5]dienones.Org Biomol Chem. 2024 May 8;22(18):3602-3605. doi: 10.1039/d4ob00274a. Org Biomol Chem. 2024. PMID: 38629922
-
Electrochemical Dearomative Spirocyclization.Chem Asian J. 2023 May 2;18(9):e202300122. doi: 10.1002/asia.202300122. Epub 2023 Mar 27. Chem Asian J. 2023. PMID: 36918354 Review.
References
-
- Hu J.-F. Fan H. Xiong J. Wu S.-B. Chem. Rev. 2011;111:5465. - PubMed
- Wu W.-T. Zhang L. You S.-L. Chem. Soc. Rev. 2016;45:1570. - PubMed
- Antunes E. M. Copp B. R. Davies-Coleman M. T. Samaai T. Nat. Prod. Rep. 2005;22:62. - PubMed
- Wang H. Luan X. Org. Biomol. Chem. 2016;14:9451. - PubMed
- Yang W.-C. Zhang M.-M. Feng J.-G. Adv. Synth. Catal. 2020;362:4446.
- Zheng Y. Tice C. M. Sing S. B. Bioorg. Med. Chem. Lett. 2014;24:3673. - PubMed
- Zuo Z. Wang J. Liu J. Wang Y. Luan X. Angew. Chem., Int. Ed. 2020;59:653. - PubMed
-
- Bariwal J. Voskressensky L. G. Van der Eycken E. V. Chem. Soc. Rev. 2018;47:3831. - PubMed
- Vitaku E. Smith D. T. Njardarson J. T. J. Med. Chem. 2014;57:10257. - PubMed
- Zheng Y. Tice C. M. Singh S. B. Bioorg. Med. Chem. Lett. 2014;24:3673. - PubMed
- Jadulco R. Edrada R. A. Ebel R. Berg A. Schaumann K. Wray V. Steube K. Proksch P. J. Nat. Prod. 2004;67:78. - PubMed
-
- Zi W. Zuo Z. Ma D. Acc. Chem. Res. 2015;48:702. - PubMed
- Bariwal J. Voskressensky L. Vandereycken E. Chem. Soc. Rev. 2018;47:3831. - PubMed
- Huang G. Yin B. Adv. Synth. Catal. 2019;361:405.
- Zhuo C.-X. Zheng C. You S.-L. Acc. Chem. Res. 2014;47:2558. - PubMed
- Wu Q.-F. Zheng C. You S.-L. Angew. Chem., Int. Ed. 2012;51:1680. - PubMed
- James M. J. O'Brien P. Taylor R. J. K. Unsworth W. P. Chem.–Eur. J. 2016;22:2856. - PubMed
-
- James M. J. Cuthbertson J. D. O'Brien P. Taylor R. J. K. Unsworth W. P. Angew. Chem., Int. Ed. 2015;54:7640. - PubMed
- Clarke A. K. James M. J. O'Brien P. Taylor R. J. K. Unsworth W. P. Angew. Chem., Int. Ed. 2016;55:13798. - PubMed
- Liddon J. T. R. Clarke A. K. Taylor R. J. K. Unsworth W. P. Org. Lett. 2016;18:6328. - PubMed
- Fedoseev P. Van der Eycken E. K. Chem. Commun. 2017;53:7732. - PubMed
- Fedoseev P. Coppola G. Ojeda G. M. Van der Eycken E. K. Chem. Commun. 2018;54:3625. - PubMed
- Ho H. E. Stephens T. C. Payne T. J. O'Brien P. Taylor R. J. K. Unsworth W. P. ACS Catal. 2019;9:504.
- Ho H. E. Pagano A. Rossi-Ashton J. A. Donald J. R. Epton R. G. Churchill J. C. James M. J. O'Brien P. Taylor R. J. K. Unsworth W. P. Chem. Sci. 2020;11:1353. - PMC - PubMed
- Zhang B. Li X. Ai Z. Zhao B. Yu Z. D Y. Org. Lett. 2022;24:390. - PubMed
- Inprung N. Ho H. E. Rossi-Ashton J. A. Epton R. G. Whitwood A. C. Lynam J. M. Taylor R. J. K. James M. J. Unsworth W. P. Org. Lett. 2022;24:668. - PubMed
- Ru G. Zhang M. Zhang T. Jiang X. Gao G. Zhu X. Wang S. Fan C. Li X. Shen W. Org. Chem. Front. 2022;9:2621.
-
- Chen Z. Lai H. Hou L. Chen T. Chem. Commun. 2020;56:179. - PubMed
- Galant L. S. Rafique J. Braga A. L. Braga F. C. Saba S. Radi R. da Rocha J. B. T. Santi C. Monsalve M. Farina M. de Bem A. F. Neurochem. Res. 2021;46:120. - PubMed
- Jin Z. Du X. Xu Y. Deng Y. Liu M. Zhao Y. Zhang B. Li X. Zhang L. Peng C. Duan Y. Yu J. Wang L. Yang K. Liu F. Jiang R. Yang X. You T. Liu X. Yang X. Bai F. Liu H. Liu X. Guddat L. W. Xu W. Xiao G. Qin C. Shi Z. Jiang H. Rao Z. Yang H. Nature. 2020;582:289. - PubMed
- Li N.-B. Xu L. Ma R. Fan Q. Li B. Qiao J. Guo R. Xu X.-H. J. Inorg. Chem. 2021;41:2723.
- Banerjee B. Koketsu M. Coord. Chem. Rev. 2017;339:104.
- Sancineto L. Mariotti A. Bagnoli L. Marini F. Desantis J. Iraci N. Santi C. Pannecouque C. Tabarrini O. J. Med. Chem. 2015;58:9601. - PubMed
LinkOut - more resources
Full Text Sources