A novel immuno-device based on the specific binding of AuNP-supported CTAB with biotinylated antibody of hyaluronic acid toward an early-stage recognition of a biomarker: a bioanalytical assay in real samples using disposal biosensor technology
- PMID: 36320526
- PMCID: PMC9533320
- DOI: 10.1039/d2ra04984h
A novel immuno-device based on the specific binding of AuNP-supported CTAB with biotinylated antibody of hyaluronic acid toward an early-stage recognition of a biomarker: a bioanalytical assay in real samples using disposal biosensor technology
Abstract
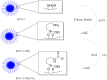
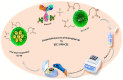
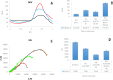
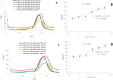
Hyaluronic Acid (HA) is a non-sulfated glycosaminoglycan, which is a potential biomarker that could be evaluated in the diagnosis of some cancers. For the first time, a novel label-free electrochemical immunosensor was developed based on modified ITO-PET (indium tin oxide-polyethylene terephthalate) electrodes for the sensitive recognition of hyaluronic acid (HA) in real samples. A disposable ITO-coated PET electrode was modified with gold nanoparticles (AuNPs) to construct a suitable substrate for the efficient immobilization of biotinylated antibodies of HA. Importantly, the encapsulation of biotinylated antibody of HA in KCC1-NH-CS2 was performed successfully, which was another innovative part of this bio-device construction. For determining the immobilization steps and optimization of the biosensor, electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) techniques were used. Furthermore, the morphological characterization of each ITO electrode surface was performed by field emission scanning electron microscopy (FESEM). Specific binding of gold nanoparticles supported CTAB to ITO-PET and its bioconjugation with the biotinylated antibody of HA was studied using the electroanalysis of the sensor performance. For the better performance of the antibody to generate an immunocomplex with HA (antigen), its encapsulation was performed, which led to the excellent behavior of the immunosensor. The proposed HA immunosensor indicated excellent reproducibility, high selectivity, and long-term stability. The HA electrochemical immunosensor performed perfectly with a wide determination range (0.078 to 160 ng mL-1) and a low limit of quantification (0.078 ng mL-1) in human plasma samples. It is recommended that the designed biosensor can be used as a diagnostic tool in clinical bioassays in the near future.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
-
- Fraser J.; Laurent T.Turnover and metabolism of hyaluronan. In Ciba Foundation Symposium 143-The Biology of Hyaluronan: The Biology of Hyaluronan: Ciba Foundation Symposium 143, 2007; Wiley Online Library: pp 41–59 - PubMed
- Marcellin E. Steen J. A. Nielsen L. K. Insight into hyaluronic acid molecular weight control. Appl. Microbiol. Biotechnol. 2014;98(16):6947–6956. - PubMed
-
- Vigetti D. Karousou E. Viola M. Deleonibus S. De Luca G. Passi A. Hyaluronan: biosynthesis and signaling. Biochim. Biophys. Acta Gen. Subj. 2014;1840(8):2452–2459. - PubMed
LinkOut - more resources
Full Text Sources

