The pursuit of polymethine fluorophores with NIR-II emission and high brightness for in vivo applications
- PMID: 36320587
- PMCID: PMC9533410
- DOI: 10.1039/d2sc03136a
The pursuit of polymethine fluorophores with NIR-II emission and high brightness for in vivo applications
Abstract
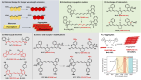
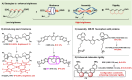
Polymethine cyanine dyes, as the most important class of organic near-infrared-II (NIR-II) fluorophores, recently received increasing attention due to their high molar extinction coefficients, intensive fluorescence brightness, and flexible wavelength tunability for fluorescent bioimaging applications. Very recently, remarkable advances have been made in the development of NIR-II polymethine fluorophores with improved optical performance, mainly including tunable fluorescence, improved brightness, improved water solubility and stability. In this review, we summarize the recent research advances in molecular tailoring design strategies of NIR-II polymethine fluorophores, and then emphasize the representative bioimaging and biosensing applications. The potential challenges and perspectives of NIR-II polymethine fluorophores in this emerging field are also discussed. This review may provide guidance and reference for further development of high-performance NIR-II polymethine fluorophores to boost their clinical translation in the future.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

