Excimer evolution hampers symmetry-broken charge-separated states
- PMID: 36320683
- PMCID: PMC9491171
- DOI: 10.1039/d2sc04387d
Excimer evolution hampers symmetry-broken charge-separated states
Abstract
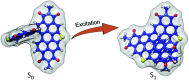
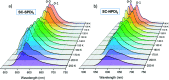
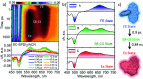
Achieving long-lived symmetry-broken charge-separated states in chromophoric assemblies is quintessential for enhanced performance of artificial photosynthetic mimics. However, the occurrence of energy trap states hinders exciton and charge transport across photovoltaic devices, diminishing power conversion efficiency. Herein, we demonstrate unprecedented excimer formation in the relaxed excited-state geometry of bichromophoric systems impeding the lifetime of symmetry-broken charge-separated states. Core-annulated perylenediimide dimers (SC-SPDI2 and SC-NPDI2) prefer a near-orthogonal arrangement in the ground state and a π-stacked foldamer structure in the excited state. The prospect of an excimer-like state in the foldameric arrangement of SC-SPDI2 and SC-NPDI2 has been rationalized by fragment-based excited state analysis and temperature-dependent photoluminescence measurements. Effective electronic coupling matrix elements in the Franck-Condon geometry of SC-SPDI2 and SC-NPDI2 facilitate solvation-assisted ultrafast symmetry-breaking charge-separation (SB-CS) in a high dielectric environment, in contrast to unrelaxed excimer formation (Ex*) in a low dielectric environment. Subsequently, the SB-CS state dissociates into an undesired relaxed excimer state (Ex) due to configuration mixing of a Frenkel exciton (FE) and charge-separated state in the foldamer structure, downgrading the efficacy of the charge-separated state. The decay rate constant of the FE to SB-CS (k FE→SB-CS) in polar solvents is 8-17 fold faster than that of direct Ex* formation (k FE→Ex*) in non-polar solvent (k FE→SB-CS≫k FE→Ex*), characterized by femtosecond transient absorption (fsTA) spectroscopy. The present investigation establishes the impact of detrimental excimer formation on the persistence of the SB-CS state in chromophoric dimers and offers the requisite of conformational rigidity as one of the potential design principles for developing advanced molecular photovoltaics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

