Molecular nanoribbon gels
- PMID: 36320686
- PMCID: PMC9491176
- DOI: 10.1039/d2sc02637f
Molecular nanoribbon gels
Abstract
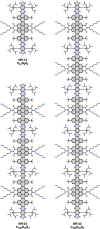
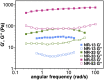
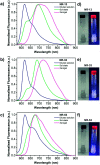
Herein, we show that twisted molecular nanoribbons with as many as 322 atoms in the aromatic core are efficient gelators capable of self-assembling into ordered π-gels with morphologies and sol-gel transitions that vary with the length of the nanoribbon. In addition, the nanoribbon gels show a red fluorescence and also pseudoconductivity values in the same range as current state-of-the-art π-gels.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
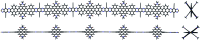


References
-
- Yao Y. F. Zhang L. Orgiu E. Samori P. Adv. Mater. 2019;31:1900599. doi: 10.1002/adma.201900599. - DOI - PubMed
- Lu F. Neal E. A. Nakanishi T. Acc. Chem. Res. 2019;52:1834–1843. doi: 10.1021/acs.accounts.9b00217. - DOI - PubMed
- Jones C. D. Steed J. W. Chem. Soc. Rev. 2016;45:6546–6596. doi: 10.1039/C6CS00435K. - DOI - PubMed
- Ghosh S. Praveen V. K. Ajayaghosh A. Annu. Rev. Mater. Res. 2016;46:235–262. doi: 10.1146/annurev-matsci-070115-031557. - DOI
- Babu S. S. Praveen V. K. Ajayaghosh A. Chem. Rev. 2014;114:1973–2129. doi: 10.1021/cr400195e. - DOI - PubMed
- Babu S. S. Prasanthkumar S. Ajayaghosh A. Angew. Chem., Int. Ed. 2012;51:1766–1776. doi: 10.1002/anie.201106767. - DOI - PubMed
-
- Hong J.-P. Um M.-C. Nam S.-R. Hong J.-I. Lee S. Chem. Commun. 2009:310–312. doi: 10.1039/B816030A. - DOI - PubMed
- Guan Y.-S. Qin Y. Sun Y. Chen J. Xu W. Zhu D. Chem. Commun. 2016;52:4648–4651. doi: 10.1039/C6CC01300G. - DOI - PubMed
- Sagade A. A. Rao K. V. Mogera U. George S. J. Datta A. Kulkarni G. U. Adv. Mater. 2013;25:559–564. doi: 10.1002/adma.201203926. - DOI - PubMed
- Guan Y.-S. Qin Y. Sun Y. Wang C. Xu W. Zhu D. Chem. Commun. 2015;51:12182–12184. doi: 10.1039/C5CC04711K. - DOI - PubMed
- Prasanthkumar S. Saeki A. Seki S. Ajayaghosh A. J. Am. Chem. Soc. 2010;132:8866–8867. doi: 10.1021/ja103685j. - DOI - PubMed
- Hollamby M. J. Karny M. Bomans P. H. H. Sommerdjik N. A. J. M. Saeki A. Seki S. Minamikawa H. Grillo I. Pauw B. R. Brown P. Eastoe J. Möhwald H. Nakanishi T. Nat. Chem. 2014;6:690. doi: 10.1038/nchem.1977. - DOI - PubMed
- López-Andarias J. Rodriguez M. J. Atienza C. López J. L. Mikie T. Casado S. Seki S. Carrascosa J. L. Martín N. J. Am. Chem. Soc. 2015;137:893–897. doi: 10.1021/ja510946c. - DOI - PubMed
- Nair V. S. Mukhopadhyay R. D. Saeki A. Seki S. Ajayaghosh A. Sci. Adv. 2016;2:e1600142. doi: 10.1126/sciadv.1600142. - DOI - PMC - PubMed
- Martín C. Kennes K. Van der Auweraer M. Hofkens J. de Miguel G. García-Frutos E. M. Adv. Funct. Mater. 2017;27:1702176. doi: 10.1002/adfm.201702176. - DOI
- Draper E. R. Walsh J. J. McDonald T. O. Zwijnenburg M. A. Cameron P. J. Cowan A. J. Adams D. J. J. Mater. Chem. C. 2014;2:5570–5575. doi: 10.1039/C4TC00744A. - DOI
- Tsai W.-W. Tevis I. D. Tayi A. S. Cui H. Stupp S. I. J. Phys. Chem. B. 2010;114:14778–14786. doi: 10.1021/jp105227p. - DOI - PubMed
- Stone D. A. Tayi A. S. Goldberger J. E. Palmer L. C. Stupp S. I. Chem. Commun. 2011;47:5702–5704. doi: 10.1039/C1CC10809C. - DOI - PubMed
- Xu J. Wang Y. Shan H. Lin Y. Chen Q. Roy V. A. L. Xu Z. ACS Appl. Mater. Interfaces. 2016;8:18991–18997. doi: 10.1021/acsami.6b04817. - DOI - PubMed
- Zhang L. Li S. Squillaci M. A. Zhong X. Yao Y. Orgiu E. Samorì P. J. Am. Chem. Soc. 2017;139:14406–14411. doi: 10.1021/jacs.7b04347. - DOI - PubMed
- Besar K. Ardoña H. A. M. Tovar J. D. Katz H. E. ACS Nano. 2015;9:12401–12409. doi: 10.1021/acsnano.5b05752. - DOI - PubMed
- Martinez-Abadia M. Antonicelli G. Saeki A. Mateo-Alonso A. Angew. Chem., Int. Ed. 2018;57:8209–8213. doi: 10.1002/anie.201804453. - DOI - PubMed
-
- Guo X. Yuan Z. Zhu Y. Li Z. Huang R. Xia Z. Zhang W. Li Y. Wang J. Angew. Chem., Int. Ed. 2019;58:16966–16972. doi: 10.1002/anie.201907972. - DOI - PubMed
- Zhu Y. Xia Z. Cai Z. Yuan Z. Jiang N. Li T. Wang Y. Guo X. Li Z. Ma S. Zhong D. Li Y. Wang J. J. Am. Chem. Soc. 2018;140:4222–4226. doi: 10.1021/jacs.8b01447. - DOI - PubMed
- Ma S. Gu J. Lin C. Luo Z. Zhu Y. Wang J. J. Am. Chem. Soc. 2020;142:16887–16893. doi: 10.1021/jacs.0c08555. - DOI - PubMed
- Cruz C. M. Márquez I. R. Castro-Fernández S. Cuerva J. M. Maçôas E. Campaña A. G. Angew. Chem., Int. Ed. 2019;58:8068–8072. doi: 10.1002/anie.201902529. - DOI - PubMed
- Cortizo-Lacalle D. Mora-Fuentes J. P. Strutyński K. Saeki A. Melle-Franco M. Mateo-Alonso A. Angew. Chem., Int. Ed. 2018;57:703–708. doi: 10.1002/anie.201710467. - DOI - PMC - PubMed
- Yan X. Cui X. Li L.-s. J. Am. Chem. Soc. 2010;132:5944–5945. doi: 10.1021/ja1009376. - DOI - PubMed
- Chen Y. Lin C. Luo Z. Yin Z. Shi H. Zhu Y. Wang J. Angew. Chem., Int. Ed. 2021;60:7796–7801. doi: 10.1002/anie.202014621. - DOI - PubMed
- Wang Y. Yin Z. Zhu Y. Gu J. Li Y. Wang J. Angew. Chem., Int. Ed. 2019;58:587–591. doi: 10.1002/anie.201811706. - DOI - PubMed
- Mora-Fuentes J. P. Riaño A. Cortizo-Lacalle D. Saeki A. Melle-Franco M. Mateo-Alonso A. Angew. Chem., Int. Ed. 2019;58:552–556. doi: 10.1002/anie.201811015. - DOI - PubMed
- Beser U. Kastler M. Maghsoumi A. Wagner M. Castiglioni C. Tommasini M. Narita A. Feng X. Müllen K. J. Am. Chem. Soc. 2016;138:4322–4325. doi: 10.1021/jacs.6b01181. - DOI - PubMed
- Simpson C. D. Brand J. D. Berresheim A. J. Przybilla L. Räder H. J. Müllen K. Chem.–Eur. J. 2002;8:1424–1429. doi: 10.1002/1521-3765(20020315)8:6<1424::AID-CHEM1424>3.0.CO;2-Z. - DOI - PubMed
- Tan Y.-Z. Yang B. Parvez K. Narita A. Osella S. Beljonne D. Feng X. Müllen K. Nat. Commun. 2013;4:2646. doi: 10.1038/ncomms3646. - DOI - PMC - PubMed
- Zhu Y. Guo X. Li Y. Wang J. J. Am. Chem. Soc. 2019;141:5511–5517. doi: 10.1021/jacs.9b01266. - DOI - PubMed
- Dubey R. K. Melle-Franco M. Mateo-Alonso A. J. Am. Chem. Soc. 2021;143:6593–6600. doi: 10.1021/jacs.1c01849. - DOI - PubMed
- Hernández-Culebras F. Melle-Franco M. Mateo-Alonso A. Angew. Chem., Int. Ed. 2022:e202205018. - PMC - PubMed
-
- Ito S. Herwig P. T. Böhme T. Rabe J. P. Rettig W. Müllen K. J. Am. Chem. Soc. 2000;122:7698–7706. doi: 10.1021/ja000850e. - DOI
- Zhi L. Wu J. Müllen K. Org. Lett. 2005;7:5761–5764. doi: 10.1021/ol052201j. - DOI - PubMed
- Kim H.-S. Lee J.-H. Kim T.-H. Okabe S. Shibayama M. Choi S.-M. J. Phys. Chem. B. 2011;115:7314–7320. doi: 10.1021/jp200882n. - DOI - PubMed
- Dou X. Pisula W. Wu J. Bodwell G. J. Müllen K. Chem.–Eur. J. 2008;14:240–249. doi: 10.1002/chem.200700921. - DOI - PubMed
- Hill J. P. Jin W. Kosaka A. Fukushima T. Ichihara H. Shimomura T. Ito K. Hashizume T. Ishii N. Aida T. Science. 2004;304:1481–1483. doi: 10.1126/science.1097789. - DOI - PubMed
- Jin W. Fukushima T. Niki M. Kosaka A. Ishii N. Aida T. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10801–10806. doi: 10.1073/pnas.0500852102. - DOI - PMC - PubMed
- Jin W. Yamamoto Y. Fukushima T. Ishii N. Kim J. Kato K. Takata M. Aida T. J. Am. Chem. Soc. 2008;130:9434–9440. doi: 10.1021/ja801179e. - DOI - PubMed
- Rao K. V. Jayaramulu K. Maji T. K. George S. J. Angew. Chem., Int. Ed. 2010;49:4218–4222. doi: 10.1002/anie.201000527. - DOI - PubMed
- Rao K. V. Datta K. K. R. Eswaramoorthy M. George S. J. Angew. Chem., Int. Ed. 2011;50:1179–1184. doi: 10.1002/anie.201006270. - DOI - PubMed
- Jain A. Rao K. V. Kulkarni C. George A. George S. J. Chem. Commun. 2012;48:1467–1469. doi: 10.1039/C1CC14990C. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

