Naphthylisoindolinone alkaloids: the first ring-contracted naphthylisoquinolines, from the tropical liana Ancistrocladus abbreviatus, with cytotoxic activity
- PMID: 36320727
- PMCID: PMC9555057
- DOI: 10.1039/d2ra05758a
Naphthylisoindolinone alkaloids: the first ring-contracted naphthylisoquinolines, from the tropical liana Ancistrocladus abbreviatus, with cytotoxic activity
Erratum in
-
Correction: Naphthylisoindolinone alkaloids: the first ring-contracted naphthylisoquinolines, from the tropical liana Ancistrocladus abbreviatus, with cytotoxic activity.RSC Adv. 2022 Oct 24;12(47):30321. doi: 10.1039/d2ra90105f. eCollection 2022 Oct 24. RSC Adv. 2022. PMID: 36337954 Free PMC article.
Abstract
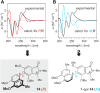
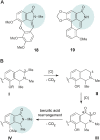
The West African liana Ancistrocladus abbreviatus is a rich source of structurally most diverse naphthylisoquinoline alkaloids. From its roots, a series of four novel representatives, named ancistrobrevolines A-D (14-17) have now been isolated, displaying an unprecedented heterocyclic ring system, where the usual isoquinoline entity is replaced by a ring-contracted isoindolinone part. Their constitutions were elucidated by 1D and 2D NMR and HR-ESI-MS. The absolute configurations at the chiral axis and at the stereogenic center were assigned by using experimental and computational electronic circular dichroism (ECD) investigations and a ruthenium-mediated oxidative degradation, respectively. For the biosynthetic origin of the isoindolinones from 'normal' naphthyltetrahydroisoquinolines, a hypothetic pathway is presented. It involves oxidative decarboxylation steps leading to a ring contraction by a benzilic acid rearrangement. Ancistrobrevolines A (14) and B (15) were found to display moderate cytotoxic effects (up to 72%) against MCF-7 breast and A549 lung cancer cells and to reduce the formation of spheroids (mammospheres) in the breast cancer cell line.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Ancistrobrevinium A, the first N-methylated, cationic naphthylisoquinoline alkaloid, from the tropical liana Ancistrocladus abbreviatus (Ancistrocladaceae).Nat Prod Res. 2024 Aug;38(15):2614-2618. doi: 10.1080/14786419.2023.2194648. Epub 2023 Mar 29. Nat Prod Res. 2024. PMID: 36987744
-
Ancistrobreveines A-D and related dehydrogenated naphthylisoquinoline alkaloids with antiproliferative activities against leukemia cells, from the West African liana Ancistrocladus abbreviatus.RSC Adv. 2019 May 20;9(28):15738-15748. doi: 10.1039/c9ra03105g. eCollection 2019 May 20. RSC Adv. 2019. PMID: 35521375 Free PMC article.
-
Correction: Naphthylisoindolinone alkaloids: the first ring-contracted naphthylisoquinolines, from the tropical liana Ancistrocladus abbreviatus, with cytotoxic activity.RSC Adv. 2022 Oct 24;12(47):30321. doi: 10.1039/d2ra90105f. eCollection 2022 Oct 24. RSC Adv. 2022. PMID: 36337954 Free PMC article.
-
Structural variety and pharmacological potential of naphthylisoquinoline alkaloids.Alkaloids Chem Biol. 2024;91:1-410. doi: 10.1016/bs.alkal.2024.03.001. Epub 2024 May 13. Alkaloids Chem Biol. 2024. PMID: 38811064 Review.
-
Asian Ancistrocladus Lianas as Creative Producers of Naphthylisoquinoline Alkaloids.Prog Chem Org Nat Prod. 2023;119:1-335. doi: 10.1007/978-3-031-10457-2_1. Prog Chem Org Nat Prod. 2023. PMID: 36587292 Review.
References
-
- Taylor C. M. Gereau R. E. Walters G. M. Ann. Mo. Bot. Gard. 2005;92:360–399.
-
- Cheek M. Kew Bull. 2000;55:871–882.
-
- Airy Shaw H. K. Kew Bull. 1949;4:68–69.
-
- Airy Shaw H. K. Kew Bull. 1950;5:147–150.
-
- Bringmann G. and Pokorny F., The naphthylisoquinoline alkaloids, in The Alkaloids, ed. G. A. Cordell, Academic Press Inc, New York, 1995, vol. 46, pp. 127–271
LinkOut - more resources
Full Text Sources

