Synthesis, electropolymerization and functionalization via click chemistry of N-alkynylated dithieno[3,2- b:2',3'- d]pyrrole
- PMID: 36320729
- PMCID: PMC9558075
- DOI: 10.1039/d2ra03265a
Synthesis, electropolymerization and functionalization via click chemistry of N-alkynylated dithieno[3,2- b:2',3'- d]pyrrole
Abstract
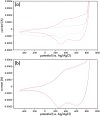
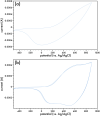
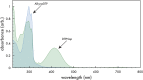
A new N-alkynylated dithieno[3,2-b:2',3'-d]pyrrole (DTP) monomer was synthesized using a Buchwald-Hartwig amination of 3,3'-dibromo-2,2'-bithiophene with pent-4-yn-1-amine. The obtained monomer was investigated for the possibility of a pre-polymerization modification via Huisgen 1,3-dipolar cycloaddition ("click") reaction with azide-containing organic compounds. The synthesized N-alkynylated DTP monomer is soluble in a number of organic solvents and reacts with organic azides via "click" reactions in mild conditions, achieving high yields. The N-alkynylated DTP monomer and its "click"-modified derivative can be electropolymerized to form polymeric films. Herein, the synthesis and characterization of a "click" modified DTP monomer, its pre-modified derivative, and their corresponding polymers are described. The developed method is a facile route to synthesize a new generation of various N-functionalized DTP homopolymers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

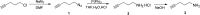




Similar articles
-
N-Benzoyl dithieno[3,2-b:2',3'-d]pyrrole-based hyperbranched polymers by direct arylation polymerization.Chem Cent J. 2017 Dec 21;11(1):135. doi: 10.1186/s13065-017-0367-0. Chem Cent J. 2017. PMID: 29270833 Free PMC article.
-
Functionalized branched EDOT-terthiophene copolymer films by electropolymerization and post-polymerization "click"-reactions.Beilstein J Org Chem. 2015 Mar 11;11:335-47. doi: 10.3762/bjoc.11.39. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 25815088 Free PMC article.
-
Facile fabrication of polymer network using click chemistry and their computational study.R Soc Open Sci. 2021 Mar 3;8(3):202056. doi: 10.1098/rsos.202056. R Soc Open Sci. 2021. PMID: 33959358 Free PMC article.
-
Electrophilic Azides for Materials Synthesis and Chemical Biology.Acc Chem Res. 2020 Apr 21;53(4):937-948. doi: 10.1021/acs.accounts.0c00046. Epub 2020 Mar 24. Acc Chem Res. 2020. PMID: 32207916 Review.
-
Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials.Acc Chem Res. 2012 Apr 17;45(4):630-40. doi: 10.1021/ar200235m. Epub 2011 Dec 8. Acc Chem Res. 2012. PMID: 22148925 Review.
References
-
- Perepichka I. F. and Perepichka D. F., Handbook of Thiophene Based Materials, Hoboken, NJ, John Wiley & Sons, 2009
-
- Rasmussen S. C. Evenson S. J. Dithieno[3,2-b:2′,3′-d]pyrrole-based materials: Synthesis and application to organic electronics. Prog. Polym. Sci. 2013;38(12):1773–1804.
-
- Nakamura M. Yang C. Zhou E. Tajima K. Hashimoto K. Polymer Bulk Heterojunction Photovoltaic Devices with Multilayer Structures Prepared by Thermal Lamination. ACS Appl. Mater. Interfaces. 2009;1(12):2703–2706. - PubMed
-
- Evenson S. J. Mumm M. J. Pokhodnya K. I. Rasmussen S. C. Highly Fluorescent Dithieno[3,2-b:2′,3′-d]pyrrole-Based Materials: Synthesis, Characterization, and OLED Device Applications. Macromolecules. 2011;44(4):835–841.
-
- Nelson T. L. Young T. M. Liu J. Mishra S. P. Belot J. A. Balliet C. L. et al. Transistor Paint: High Mobilities in Small Bandgap Polymer Semiconductor Based on the Strong Acceptor, Diketopyrrolopyrrole and Strong Donor, Dithienopyrrole. Adv. Mater. 2010;22(41):4617–4621. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

