1,4-Dihydropyridine: synthetic advances, medicinal and insecticidal properties
- PMID: 36320730
- PMCID: PMC9555063
- DOI: 10.1039/d2ra04589c
1,4-Dihydropyridine: synthetic advances, medicinal and insecticidal properties
Abstract
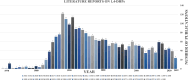
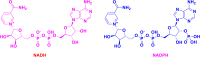
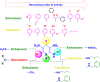
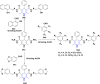
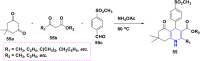
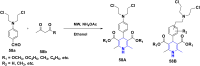
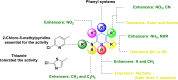
1,4-Dihydropyridine (1,4-DHP) is one of the foremost notable organic scaffolds with diverse pharmaceutical applications. This study will highlight recent accomplishments in the construction of 1,4-DHP with structural and functional modifications using multi-component one-pot and green synthetic methodologies. The various intrinsic therapeutic applications, ranging from calcium channel blocker, anti-oxidative, anticancer, anti-inflammatory, anti-microbial, anti-hypertensive, anti-diabetic, anticoagulants, anti-cholinesterase, neuro-protective, and other miscellaneous activities, have been summarized with a focus on their structure-activity relationship (SAR) investigations. In addition, the insecticidal properties have been collated and discussed. Researchers in the fields of medicinal chemistry and drug development will find the summarized conclusions of this study incredibly informative, instructional, and valuable.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

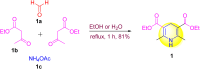

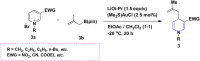
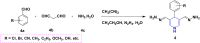
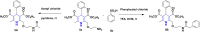
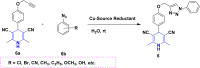

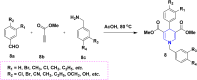


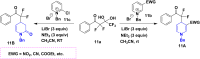

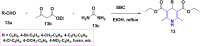
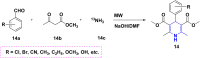

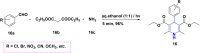
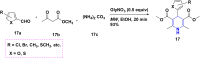

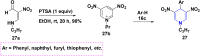
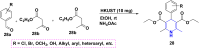
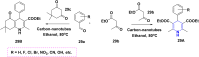
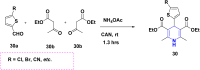
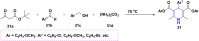

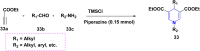
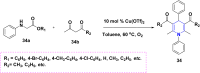

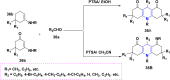
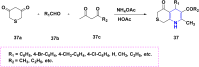
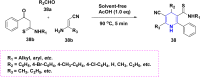
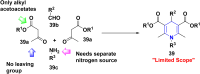
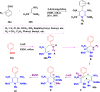
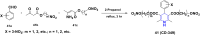
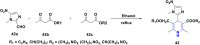
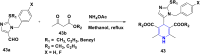
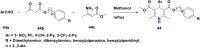
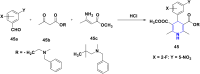
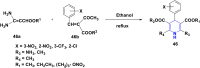
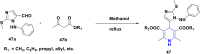
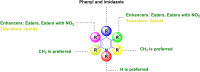
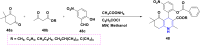
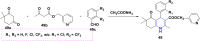
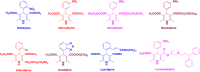
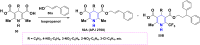
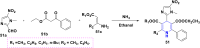
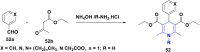
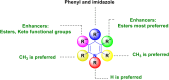




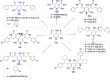
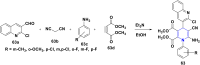
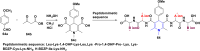


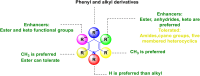


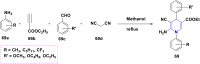

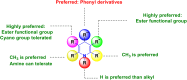
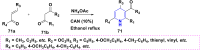


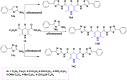
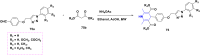
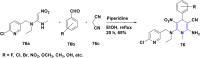
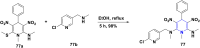
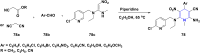
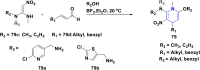
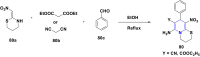
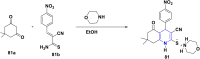
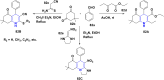
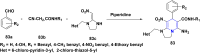
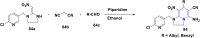
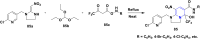
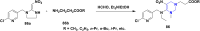


References
-
- Maria Assunta C. Daniela I. Roberto R. Salvatore V. G. Laura L. Curr. Med. Chem. 2019;26:7166–7195.
-
- Khan E. ChemistrySelect. 2021;6:3041–3064.
-
- Eisner U. Kuthan J. Chem. Rev. 1972;72:1–42.
-
- Wan J. P. Pan Y. Mini-Rev. Med. Chem. 2012;12:337–349. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

