Amyloid fibrillation of the glaucoma associated myocilin protein is inhibited by epicatechin gallate (ECG)
- PMID: 36320765
- PMCID: PMC9562371
- DOI: 10.1039/d2ra05061g
Amyloid fibrillation of the glaucoma associated myocilin protein is inhibited by epicatechin gallate (ECG)
Erratum in
-
Correction: Amyloid fibrillation of the glaucoma associated myocilin protein is inhibited by epicatechin gallate (ECG).RSC Adv. 2022 Dec 23;13(1):720. doi: 10.1039/d2ra90131e. eCollection 2022 Dec 19. RSC Adv. 2022. PMID: 36605666 Free PMC article.
Abstract
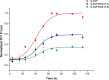
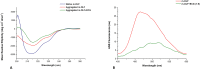
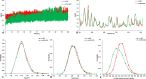
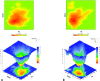
Inherited glaucoma is a recent addition to the inventory of diseases arising due to protein misfolding. Mutations in the olfactomedin (OLF) domain of myocilin are the most common genetic cause behind this disease. Disease associated variants of m-OLF are predisposed to misfold and aggregate in the trabecular meshwork (TM) tissue of the eye. In recent years, the nature of these aggregates was revealed to exhibit the hallmarks of amyloids. Amyloid aggregates are highly stable structures that are formed, often with toxic consequences in a number of debilitating diseases. In spite of its clinical relevance the amyloidogenic nature of m-OLF has not been studied adequately. Here we have studied the amyloid fibrillation of m-OLF and report ECG as an inhibitor against it. Using biophysical and biochemical assays, coupled with advanced microscopic evaluations we show that ECG binds and stabilizes native m-OLF and thus prevents its aggregation into amyloid fibrils. Furthermore, we have used REMD simulations to delineate the stabilizing effects of ECG on the structure of m-OLF. Collectively, we report ECG as a molecular scaffold for designing and testing of novel inhibitors against m-OLF amyloid fibrillation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

