Exploring the adsorption ability with sensitivity and reactivity of C12-B6N6, C12-Al6N6, and B6N6-Al6N6 heteronanocages towards the cisplatin drug: a DFT, AIM, and COSMO analysis
- PMID: 36320781
- PMCID: PMC9578514
- DOI: 10.1039/d2ra04011e
Exploring the adsorption ability with sensitivity and reactivity of C12-B6N6, C12-Al6N6, and B6N6-Al6N6 heteronanocages towards the cisplatin drug: a DFT, AIM, and COSMO analysis
Abstract
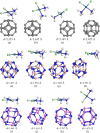
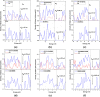
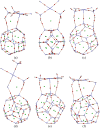
The DFT study on the adsorption behaviour of the C24, B12N12, and Al12N12 nanocages and their heteronanocages towards the anticancer drug cisplatin (CP) was performed in gas and water media. Among the three pristine nanocages, Al12N12 exhibited high adsorption energy ranging from -1.98 to -1.63 eV in the gas phase and -1.47 to -1.39 eV in water media. However, their heterostructures C12-Al6N6 and B6N6-Al6N6 showed higher interaction energies (-2.22 eV and -2.14 eV for C12-Al6N6 and B6N6-Al6N6) with a significant amount of charge transfer. Noteworthy variations in electronic properties were confirmed by FMO analysis and DOS spectra analysis after the adsorption of the cisplatin drug on B12N12 and B6N6-Al6N6 nanocages. Furthermore, an analysis of quantum molecular descriptors unveiled salient decrement in global hardness and increments in electrophilicity index and global softness occurred after the adsorption of CP on B12N12 and B6N6-Al6N6. On the other hand, the above-mentioned fluctuations are not so noteworthy in the case of the adsorption of CP on Al12N12, C12-B6N6, and C12-Al6N6. Concededly, energy calculation, FMO analysis, ESP map, DOS spectra, quantum molecular descriptors, dipole moment, COSMO surface analysis, QTAIM analysis, and work function analysis predict that B12N12 and B6N6-Al6N6 nanocages exhibit high sensitivity towards CP drug molecules.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Understanding the adsorption performance of hetero-nanocages (C12-B6N6, C12-Al6N6, and B6N6-Al6N6) towards hydroxyurea anticancer drug: a comprehensive study using DFT.Nanoscale Adv. 2024 Oct 3;6(23):5988-6007. doi: 10.1039/d4na00472h. Online ahead of print. Nanoscale Adv. 2024. PMID: 39372438 Free PMC article.
-
Advances in Selective Detection of Cadaverine by Electronic, Optical, and Work Function Sensors Based on Cu-Modified B12N12 and Al12N12 Nanocages: A Density Functional Theory (DFT) Study.Langmuir. 2024 Nov 5;40(44):23310-23323. doi: 10.1021/acs.langmuir.4c02699. Epub 2024 Oct 22. Langmuir. 2024. PMID: 39435972 Free PMC article.
-
Adsorption of drugs on B12N12 and Al12N12 nanocages.RSC Adv. 2024 Oct 8;14(43):31756-31767. doi: 10.1039/d4ra05586a. eCollection 2024 Oct 1. RSC Adv. 2024. PMID: 39380648 Free PMC article.
-
Comparative study of the therapeutic potential of C24, C32, B12N12, and B16N16 nanocages as drug delivery carriers for delivering an erlotinib derivative: DFT and QTAIM investigations.Nanoscale. 2025 May 9;17(18):11413-11425. doi: 10.1039/d4nr05393a. Nanoscale. 2025. PMID: 40242881
-
Adsorption of Molnupiravir anti-COVID-19 drug over B12N12 and Al12N12 nanocarriers: a DFT study.J Biomol Struct Dyn. 2023;41(22):12923-12937. doi: 10.1080/07391102.2023.2169763. Epub 2023 Jan 23. J Biomol Struct Dyn. 2023. PMID: 36688358
Cited by
-
Phosphorus-doped T-graphene nanocapsule toward O3 and SO2 gas sensing: a DFT and QTAIM analysis.Sci Rep. 2024 Feb 12;14(1):3467. doi: 10.1038/s41598-024-54110-z. Sci Rep. 2024. PMID: 38342938 Free PMC article.
-
Al and Ti-doped black phosphorus as sensitive materials for adsorption of HF and H2S toxic gases: an ab initio study.RSC Adv. 2025 Aug 13;15(35):28703-28720. doi: 10.1039/d5ra04844c. eCollection 2025 Aug 11. RSC Adv. 2025. PMID: 40861972 Free PMC article.
-
First-principles investigations of As-doped tetragonal boron nitride nanosheets for toxic gas sensing applications.Nanoscale Adv. 2024 Nov 21;7(1):354-369. doi: 10.1039/d4na00739e. eCollection 2024 Dec 17. Nanoscale Adv. 2024. PMID: 39629350 Free PMC article.
-
A DFT and QTAIM insight into ethylene oxide adsorption on the surfaces of pure and metal-decorated inorganic fullerene-like nanoclusters.Heliyon. 2023 Aug 25;9(9):e19407. doi: 10.1016/j.heliyon.2023.e19407. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809619 Free PMC article.
-
Understanding the adsorption performance of hetero-nanocages (C12-B6N6, C12-Al6N6, and B6N6-Al6N6) towards hydroxyurea anticancer drug: a comprehensive study using DFT.Nanoscale Adv. 2024 Oct 3;6(23):5988-6007. doi: 10.1039/d4na00472h. Online ahead of print. Nanoscale Adv. 2024. PMID: 39372438 Free PMC article.
References
-
- Alderden R. a. Hall M. D. Hambley T. W. J. Chem. Educ. 2006;83:728–734.
-
- Topps J. Elliott R. C. Nature. 1965;205:498–499. - PubMed
-
- Go R. S. Adjei A. A. J. Clin. Oncol. 1999;17:409–422. - PubMed
-
- Perveen M. Nazir S. Arshad A. W. Khan M. I. Shamim M. Ayub K. Khan M. A. Iqbal J. Biophys. Chem. 2020;267:106461. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

