Withholding methotrexate after vaccination with ChAdOx1 nCov19 in patients with rheumatoid or psoriatic arthritis in India (MIVAC I and II): results of two, parallel, assessor-masked, randomised controlled trials
- PMID: 36320825
- PMCID: PMC9612848
- DOI: 10.1016/S2665-9913(22)00228-4
Withholding methotrexate after vaccination with ChAdOx1 nCov19 in patients with rheumatoid or psoriatic arthritis in India (MIVAC I and II): results of two, parallel, assessor-masked, randomised controlled trials
Abstract
Background: There is a necessity for an optimal COVID-19 vaccination strategy for vulnerable population groups, including people with autoimmune inflammatory arthritis on immunosuppressants such as methotrexate, which inhibit vaccine-induced immunity against SARS-CoV-2. Thus, we aimed to assess the effects of withholding methotrexate for 2 weeks after each dose of ChAdOx1 nCov-19 (Oxford-AstraZeneca) vaccine (MIVAC I) or only after the second dose of vaccine (MIVAC II) compared with continuation of methotrexate, in terms of post-vaccination antibody titres and disease flare rates.
Methods: MIVAC I and II were two parallel, independent, assessor-masked, randomised trials. The trials were done at a single centre (Dr Shenoy's Centre for Arthritis and Rheumatism Excellence; Kochi, India) in people with either rheumatoid arthritis or psoriatic arthritis with stable disease activity, who had been on a fixed dose of methotrexate for the preceding 6 weeks. Those with previous COVID-19 or who were positive for anti-SARS-CoV-2 nucleocapsid antibodies were excluded from the trials. People on high-dose corticosteroids and rituximab were also excluded, whereas other disease-modifying antirheumatic drugs were allowed. In MIVAC I, participants were randomly assigned (1:1) to stop methotrexate treatment for 2 weeks after each vaccine dose or to continue methotrexate treatment. In MIVAC II, participants who had continued methotrexate during the first dose of vaccine were randomly assigned (1:1) to withhold methotrexate for 2 weeks after the second dose of vaccine or to continue to take methotrexate. The treating physician was masked to the group assignments. The primary outcome for both MIVAC I and MIVAC II was the titre (absolute value) of anti-receptor binding domain (RBD) antibody measured 4 weeks after the second dose of vaccine. All analyses were done per protocol. The trials were registered with the Clinical Trials Registry- India, number CTRI/2021/07/034639 (MIVAC I) and CTRI/2021/07/035307 (MIVAC II).
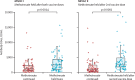
Findings: Between July 6 and Dec 15, 2021, participants were recruited to the trials. In MIVAC I, 250 participants were randomly assigned and 158 completed the study as per the protocol (80 in the methotrexate hold group and 78 in the control group; 148 [94%] were women and 10 [6%] were men). The median post-vaccination antibody titres in the methotrexate hold group were significantly higher compared with the control group (2484·0 IU/mL, IQR 1050·0-4388·8 vs 1147·5 IU/mL, 433·5-2360·3; p=0·0014). In MIVAC II, 178 participants were randomly assigned and 157 completed the study per protocol (76 in the methotrexate hold group and 81 in the control group; 135 [86%] were women and 22 [14%] were men). The methotrexate hold group had higher post-vaccination antibody titres compared with the control group (2553·5 IU/ml, IQR 1792·5-4823·8 vs 990·5, 356·1-2252·5; p<0·0001). There were no reports of any serious adverse events during the trial period.
Interpretation: Withholding methotrexate after both ChAdOx1 nCov-19 vaccine doses and after only the second dose led to higher anti-RBD antibody titres compared with continuation of methotrexate. However, withholding methotrexate only after the second vaccine dose resulted in a similar humoral response to holding methotrexate after both vaccine doses, without an increased risk of arthritis flares. Hence, interruption of methotrexate during the second dose of ChAdOx1 nCov-19 vaccine appears to be a safe and effective strategy to improve the antibody response in patients with rheumatoid or psoriatic arthritis.
Funding: Indian Rheumatology Association.
© 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
SA has received honorarium as a speaker from Pfizer, DrReddy's, Cipla, and Novartis, outside the current work. All other authors declare no competing interests.
Figures
Similar articles
-
Considerations for Pharmacologic Management of Rheumatoid Arthritis in the COVID-19 Era: a Narrative Review.Curr Rheumatol Rep. 2023 Nov;25(11):236-245. doi: 10.1007/s11926-023-01111-y. Epub 2023 Aug 19. Curr Rheumatol Rep. 2023. PMID: 37597102 Review.
-
Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial.Lancet Respir Med. 2022 Sep;10(9):840-850. doi: 10.1016/S2213-2600(22)00186-2. Epub 2022 Jun 27. Lancet Respir Med. 2022. PMID: 35772416 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine in children aged 6-17 years: a preliminary report of COV006, a phase 2 single-blind, randomised, controlled trial.Lancet. 2022 Jun 11;399(10342):2212-2225. doi: 10.1016/S0140-6736(22)00770-X. Lancet. 2022. PMID: 35691324 Free PMC article. Clinical Trial.
-
Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002).Lancet. 2021 Sep 11;398(10304):981-990. doi: 10.1016/S0140-6736(21)01699-8. Epub 2021 Sep 1. Lancet. 2021. PMID: 34480858 Free PMC article.
-
New-Onset Arthritis Following COVID-19 Vaccination: A Systematic Review of Case Reports.Vaccines (Basel). 2023 Mar 15;11(3):665. doi: 10.3390/vaccines11030665. Vaccines (Basel). 2023. PMID: 36992249 Free PMC article. Review.
Cited by
-
Correlates of breakthrough COVID-19 in vaccinated patients with systemic sclerosis: survival analysis from a multicentre international patient-reported survey.Rheumatol Int. 2024 Jan;44(1):89-97. doi: 10.1007/s00296-023-05433-z. Epub 2023 Sep 5. Rheumatol Int. 2024. PMID: 37668836
-
Effect of methotrexate hold on COVID-19 vaccine response in the patients with autoimmune inflammatory disorders: a systematic review and meta-analysis.Clin Rheumatol. 2024 Jul;43(7):2203-2214. doi: 10.1007/s10067-024-07013-3. Epub 2024 May 27. Clin Rheumatol. 2024. PMID: 38802670
-
Considerations for Pharmacologic Management of Rheumatoid Arthritis in the COVID-19 Era: a Narrative Review.Curr Rheumatol Rep. 2023 Nov;25(11):236-245. doi: 10.1007/s11926-023-01111-y. Epub 2023 Aug 19. Curr Rheumatol Rep. 2023. PMID: 37597102 Review.
-
Humoral Immunogenicity of mRNA Booster Vaccination after Heterologous CoronaVac-ChAdOx1 nCoV-19 or Homologous ChAdOx1 nCoV-19 Vaccination in Patients with Autoimmune Rheumatic Diseases: A Preliminary Report.Vaccines (Basel). 2023 Feb 24;11(3):537. doi: 10.3390/vaccines11030537. Vaccines (Basel). 2023. PMID: 36992120 Free PMC article.
-
Immunogenicity and safety of mixed COVID-19 vaccine regimens in patients with immune-mediated inflammatory diseases: a single-centre prospective cohort study.BMJ Open. 2023 May 30;13(5):e071397. doi: 10.1136/bmjopen-2022-071397. BMJ Open. 2023. PMID: 37253487 Free PMC article.
References
-
- CDC People with certain medical conditions. Feb 25, 2022. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-...
-
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. - PubMed
-
- Bitoun S, Nocturne G, Ly B, et al. Methotrexate and BAFF interaction prevents immunization against TNF inhibitors. Ann Rheum Dis. 2018;77:1463–1470. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

