Synthesis and hybridizing properties of P-stereodefined chimeric [PS]-{DNA:RNA} and [PS]-{DNA:(2'-OMe)-RNA} oligomers
- PMID: 36320848
- PMCID: PMC9491215
- DOI: 10.1039/d2ra04855h
Synthesis and hybridizing properties of P-stereodefined chimeric [PS]-{DNA:RNA} and [PS]-{DNA:(2'-OMe)-RNA} oligomers
Abstract
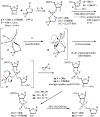
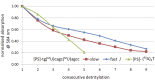
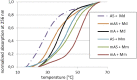
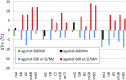
Oxathiaphospholane derivatives of 2'-OMe-ribonucleosides and 2'-O-TBDMS-ribonucleosides (MN-OTP and TN-OTP, respectively; nucleobase protected) were synthesized and separated into pure P-diastereomers. X-ray analysis showed the R P absolute configuration of the phosphorus atom in the fast-eluting diastereomer of TA-OTP. The fast- and slow-eluting P-diastereomers of MN-OTP and TN-OTP were used in the solid-phase synthesis of phosphorothioate dinucleotides (MNPST and NPST, respectively), which were subsequently hydrolyzed with R P-selective phosphodiesterase svPDE and S P-selective nuclease P1 to determine the absolute configuration of the phosphorus atoms. P-Stereodefined phosphorothioate ([PS]) 10-mer chimeric oligomers [PS]-{DNA:(2'-OMe)-RNA} and isosequential [PS]-{DNA:RNA} containing two MNPS or NPS units were synthesized. Melting experiments performed for their complexes with Watson-Crick paired DNA matrix showed that MNPS or NPS units decrease the thermal stability of the duplexes (ΔT m = -0.5 ÷ -5.5 °C per modification) regardless of the absolute configuration of the P-atoms. When the (2'-OMe)-RNA matrix was used an increase in T m was noted in all cases (ΔT m = +1 ÷ +7 °C per modification). The changes in thermal stability of the duplexes formed by [PS]-chimeras with DNA and (2'-OMe)-RNA matrices do not correlate with the absolute configuration of the phosphorus atoms.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Thermal stability and conformation of antiparallel duplexes formed by P-stereodefined phosphorothioate DNA/LNA chimeric oligomers with DNA and RNA matrices.Org Biomol Chem. 2015 Oct 21;13(39):10032-40. doi: 10.1039/c5ob01474c. Org Biomol Chem. 2015. PMID: 26293357
-
Homopurine RP-stereodefined phosphorothioate analogs of DNA with hampered Watson-Crick base pairings form Hoogsteen paired parallel duplexes with (2'-OMe)-RNAs.Org Biomol Chem. 2019 May 8;17(18):4611-4620. doi: 10.1039/c8ob03112f. Org Biomol Chem. 2019. PMID: 31017142
-
P-Stereodefined phosphorothioate analogs of glycol nucleic acids-synthesis and structural properties.RSC Adv. 2018 Jul 11;8(44):24942-24952. doi: 10.1039/c8ra05568h. eCollection 2018 Jul 9. RSC Adv. 2018. PMID: 35542141 Free PMC article.
-
Synthesis of phosphorothioate oligonucleotides with stereodefined phosphorothioate linkages.Curr Protoc Nucleic Acid Chem. 2003 Oct;Chapter 4:Unit 4.17. doi: 10.1002/0471142700.nc0417s14. Curr Protoc Nucleic Acid Chem. 2003. PMID: 18428907 Review.
-
Oligonucleotide n3'-->p5' phosphoramidates and thio-phoshoramidates as potential therapeutic agents.Chem Biodivers. 2010 Mar;7(3):477-93. doi: 10.1002/cbdv.200900187. Chem Biodivers. 2010. PMID: 20232321 Review.
Cited by
-
10th anniversary of discovering cGAMP: synthesis and beyond.Org Chem Front. 2023 Feb 21;10(4):1086-1098. doi: 10.1039/d2qo02033e. Epub 2023 Jan 25. Org Chem Front. 2023. PMID: 37144138 Free PMC article.
-
An efficient alternative to DBU in the oxathiaphospholane (OTP) method for the solid phase synthesis of P-stereodefined phosphorothioate analogs.RSC Adv. 2024 Jul 4;14(29):21174-21179. doi: 10.1039/d4ra02833c. eCollection 2024 Jun 27. RSC Adv. 2024. PMID: 38966816 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

