The effect of Bacillus cereus LV-1 on the crystallization and polymorphs of calcium carbonate
- PMID: 36320852
- PMCID: PMC9490766
- DOI: 10.1039/d2ra04254a
The effect of Bacillus cereus LV-1 on the crystallization and polymorphs of calcium carbonate
Abstract
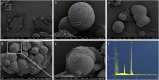
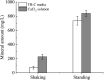
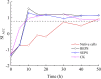
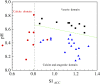
The study of CaCO3 polymorphism is of great significance for understanding the mechanism of carbonate mineralization induced by bacteria and the genesis of carbonate rock throughout geological history. To investigate the effect of bacteria and shear force on CaCO3 precipitation and polymorphs, biomineralization experiments with Bacillus cereus strain LV-1 were conducted under the standing and shaking conditions. The results show that LV-1 induced the formation of calcite and vaterite under the standing and shaking conditions, respectively. However, the results of mineralization in the media and the CaCl2 solution under both kinetic conditions suggest the shear force does not affect the polymorphs of calcium carbonate in abiotic systems. Further, mineralization experiments with bacterial cells and extracellular polymeric substances (EPS) were performed under the standing conditions. The results reveal that bacterial cells, bound EPS (BEPS), and soluble EPS (SEPS) are favorable to the formation of spherical, imperfect rhombohedral, and perfect rhombohedral minerals, respectively. The increase in the pH value and saturation index (SI) caused by LV-1 metabolism under the shear force played key roles in controlling vaterite precipitation, whereas bacterial cells and EPS do not play roles in promoting vaterite formation. Furthermore, we suggest that vaterite formed if pH > 8.5 and SIACC > 0.8, while calcite formed if pH was between 8.0-9.0 and SIACC < 0.8. Bacterial cells and BEPS are the main factors affecting CaCO3 morphologies in the mineralization process of LV-1. This may provide a deeper insight into the regulation mechanism of the polymorphs and morphologies during bacterially induced carbonate mineralization.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors report no declarations of interest.
Figures





References
-
- Li H. Yao Q. Z. Wang F. P. Huang Y. R. Fu S. Q. Zhou G. T. Geochim. Cosmochim. Acta. 2019;256:35–48.
-
- Qin W. Wang C. Y. Ma Y. X. Shen M. J. Li J. Jiao K. Tay F. R. Niu L. N. Adv. Mater. 2020;32:e1907833. - PubMed
-
- Han Z. Wang J. Zhao H. Tucker M. E. Zhao Y. Wu G. Zhou J. Yin J. Zhang H. Zhang X. Yan H. Minerals. 2019;9:218.
LinkOut - more resources
Full Text Sources

