Nano zero-valent iron loaded corn-straw biochar for efficient removal of hexavalent chromium: remediation performance and interfacial chemical behaviour
- PMID: 36320854
- PMCID: PMC9534316
- DOI: 10.1039/d2ra04650d
Nano zero-valent iron loaded corn-straw biochar for efficient removal of hexavalent chromium: remediation performance and interfacial chemical behaviour
Abstract
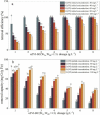
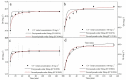
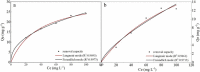
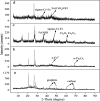
To improve the poor stability of nano zero-valent iron (nZVI), corn-straw biochar (BC) was used as a support for the synthesis of composites of nZVI-biochar (nZVI/BC) in different mass ratios. After a thorough characterization, the obtained nZVI/BC composite was used to remove hexavalent chromium [Cr(vi)] in an aquatic system under varying conditions including composite amount, Cr(vi) concentration, and pH. The obtained results show that the treatment efficiency varied in the following order: nZVI-BC (1 : 3) > nZVI-BC (1 : 5) > nZVI alone > BC alone. This order indicates the higher efficiency of composite material and the positive effect of nZVI content in the composite. Similarly, the composite dosage and Cr(vi) concentration had significant effects on the removal performance and 2 g L-1 and 6 g L-1 were considered to be the optimum dose at a Cr(vi) concentration of 20 mg L-1 and 100 mg L-1, respectively. The removal efficiency was maximum (100%) at pH 2 whereas solution pH increased significantly after the reaction (from 2 to 4.13). The removal kinetics of Cr(vi) was described by a pseudo-second-order model which indicated that the removal process was mainly controlled by the rate of chemical adsorption. The thermodynamics was more in line with the Freundlich model which indicated that the removal was multi-molecular layer adsorption. TEM-EDS, XRD, and XPS were applied to characterize the crystal lattice and structural changes of the material to specify the interfacial chemical behaviour on the agent surface. These techniques demonstrate that the underlying mechanisms of Cr(vi) removal include adsorption, chemical reduction-oxidation reaction, and co-precipitation on the surface of the nZVI-BC composite. The results indicated that the corn-straw BC as a carrier material highly improved Cr(vi) removal performance of nZVI and offered better utilization of the corn straw.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that this work is original and has not been published elsewhere, nor has been submitted to any other journal and all the authors have agreed to submit the manuscript in ‘RSC Advances’. Also, we declare that there are no conflicts of interest.
Figures






Similar articles
-
Integrated green complexing agent and biochar modified nano zero-valent iron for hexavalent chromium removal: A characterisation and performance study.Sci Total Environ. 2022 Aug 15;834:155080. doi: 10.1016/j.scitotenv.2022.155080. Epub 2022 Apr 8. Sci Total Environ. 2022. PMID: 35398438
-
Preparation of Cellulose-Grafted Acrylic Acid Stabilized Jujube Branch Biochar-Supported Nano Zero-Valent Iron Composite for Cr(VI) Removal from Water.Nanomaterials (Basel). 2025 Mar 14;15(6):441. doi: 10.3390/nano15060441. Nanomaterials (Basel). 2025. PMID: 40137614 Free PMC article.
-
Green synthesis of nZVI-modified biochar significantly enhanced the removal of Cr(VI) from aqueous solution.Environ Sci Pollut Res Int. 2024 May;31(23):33993-34009. doi: 10.1007/s11356-024-33553-x. Epub 2024 May 2. Environ Sci Pollut Res Int. 2024. PMID: 38696011
-
Biochar-supported nZVI for the removal of Cr(VI) from soil and water: Advances in experimental research and engineering applications.J Environ Manage. 2022 Aug 15;316:115211. doi: 10.1016/j.jenvman.2022.115211. Epub 2022 May 11. J Environ Manage. 2022. PMID: 35561491 Review.
-
Remediation of Cr(VI) Polluted Groundwater Using Zero-Valent Iron Composites: Preparation, Modification, Mechanisms, and Environmental Implications.Molecules. 2024 Dec 2;29(23):5697. doi: 10.3390/molecules29235697. Molecules. 2024. PMID: 39683856 Free PMC article. Review.
Cited by
-
Assessment of biochar filter application in improving chromium stress tolerance and plant physiology in Chinese cabbage (Brassica rapa) under a flow-through water setup.BMC Biotechnol. 2025 Jul 19;25(1):74. doi: 10.1186/s12896-025-01010-3. BMC Biotechnol. 2025. PMID: 40684162 Free PMC article.
-
Cost-effective core@shell structured zero-valent iron nanoparticles @ magnetic (nZVI@Fe3O4) for Cr(vi) removal from aqueous solutions: preparation by disproportionation of Fe(ii).RSC Adv. 2023 Sep 8;13(38):26983-26994. doi: 10.1039/d3ra03133k. eCollection 2023 Sep 4. RSC Adv. 2023. PMID: 37692341 Free PMC article.
-
Chromium toxicity, speciation, and remediation strategies in soil-plant interface: A critical review.Front Plant Sci. 2023 Jan 13;13:1081624. doi: 10.3389/fpls.2022.1081624. eCollection 2022. Front Plant Sci. 2023. PMID: 36714741 Free PMC article. Review.
References
-
- Tang J. Zhang L. Zhang J. Ren L. Zhou Y. Zheng Y. Luo L. Yang Y. Huang H. Chen A. Sci. Total Environ. 2020;701:134751. - PubMed
-
- Li S. Yang F. Li J. Cheng K. Sci. Total Environ. 2020;746:141037. - PubMed
-
- Baragaño D. Forján R. Fernández B. Ayala J. Afif E. Gallego J. L. R. Environ. Sci. Pollut. Res. 2020;27:33681–33691. - PubMed
-
- Chatterjee J. Kumar P. Sharma P. N. Tewari R. K. Indian J. Plant Physiol. 2015;20:1–7.
LinkOut - more resources
Full Text Sources
Other Literature Sources

