Biocompatibility Studies on a Collagen-Hydroxyapatite Biomaterial
- PMID: 36320879
- PMCID: PMC9590366
- DOI: 10.12865/CHSJ.48.02.12
Biocompatibility Studies on a Collagen-Hydroxyapatite Biomaterial
Abstract
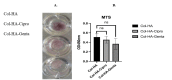
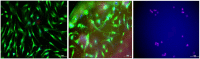
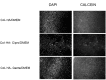
The current treatment of osteomyelitis includes systemic antibiotic therapy and a debridement procedure of the formed biofilm and necrotic tissue. Moreover, cements and three-dimensional scaffolds are used both for the delivery of therapeutic agents and as fillers for bone defects. The aim of our research was to test, on cellular cultures, the biocompatibility of a previously synthesized microporous biocomposite containing hydroxyapatite and a collagen matrix including a therapeutic agent (ciprofloxacin and gentamicin). The scaffold was obtained by direct mineralization namely co-precipitation of hydroxyapatite on a collagen matrix.
Keywords: Biocomposite; biocompatibility; collagen-hydroxyapatite.
Copyright © 2014, Medical University Publishing House Craiova.
Conflict of interest statement
None to declare.
Figures






Similar articles
-
Development of Hybrid Implantable Local Release Systems Based on PLGA Nanoparticles with Applications in Bone Diseases.Polymers (Basel). 2024 Oct 31;16(21):3064. doi: 10.3390/polym16213064. Polymers (Basel). 2024. PMID: 39518273 Free PMC article.
-
Biocompatible antibiotic-loaded mesoporous silica/bioglass/collagen-based scaffolds as bone drug delivery systems.Int J Pharm. 2023 Oct 15;645:123408. doi: 10.1016/j.ijpharm.2023.123408. Epub 2023 Sep 11. Int J Pharm. 2023. PMID: 37703959
-
Single stage treatment of diabetic calcaneal osteomyelitis with an absorbable gentamicin-loaded calcium sulphate/hydroxyapatite biocomposite: The Silo technique.Foot (Edinb). 2018 Mar;34:40-44. doi: 10.1016/j.foot.2017.11.011. Epub 2017 Nov 23. Foot (Edinb). 2018. PMID: 29278835
-
Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration-A Review.Materials (Basel). 2020 Apr 9;13(7):1748. doi: 10.3390/ma13071748. Materials (Basel). 2020. PMID: 32283608 Free PMC article. Review.
-
Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration.Mater Sci Eng C Mater Biol Appl. 2020 May;110:110698. doi: 10.1016/j.msec.2020.110698. Epub 2020 Jan 29. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32204012 Free PMC article. Review.
Cited by
-
Polysaccharide Hydroxyapatite (Nano)composites and Their Biomedical Applications: An Overview of Recent Years.ACS Omega. 2024 Jul 2;9(28):30035-30070. doi: 10.1021/acsomega.4c02170. eCollection 2024 Jul 16. ACS Omega. 2024. PMID: 39035931 Free PMC article. Review.
-
PLGA-The Smart Biocompatible Polimer: Kinetic Degradation Studies and Active Principle Release.Curr Health Sci J. 2023 Jul-Sep;49(3):416-422. doi: 10.12865/CHSJ.49.03.15. Epub 2023 Sep 30. Curr Health Sci J. 2023. PMID: 38314224 Free PMC article.
-
Revolutionizing bone healing: the role of 3D models.Cell Regen. 2025 Mar 21;14(1):7. doi: 10.1186/s13619-025-00225-1. Cell Regen. 2025. PMID: 40113735 Free PMC article. Review.
References
-
- Ferguson J, Alexander M, Bruce S, O'Connell M, Beecroft S, McNally M. A retrospective cohort study comparing clinical outcomes and healthcare resource utilisation in patients undergoing surgery for osteomyelitis in England: a case for reorganising orthopaedic infection services. J Bone Jt Infect. 2021;28;6(5):151–163. - PMC - PubMed
-
- Thirumalai J, editor. Hydroxyapatite-Advances in Composite Nanomaterials,Biomedical Applications and Its Technological Facets. Rijeka: InTech; 2018. pp. 109–109.
-
- Ciocilteu MV, Mocanu AG, Mocanu A, Ducu C, Nicolaescu OE, Manda VC, Turcu-Stiolica A, Nicolicescu C, Melinte R, Balasoiu M, Croitoru O, Neamtu J. Hydroxyapatite-ciprofloxacin delivery system: Synthesis, characterisation and antibacterial activity. Acta Pharm. 2018;68(2):129–144. - PubMed
LinkOut - more resources
Full Text Sources
