A peptide-crosslinking approach identifies HSPA8 and PFKL as selective interactors of an actin-derived peptide containing reduced and oxidized methionine
- PMID: 36320891
- PMCID: PMC9533414
- DOI: 10.1039/d2cb00183g
A peptide-crosslinking approach identifies HSPA8 and PFKL as selective interactors of an actin-derived peptide containing reduced and oxidized methionine
Abstract
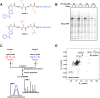
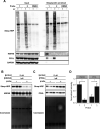
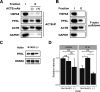
The oxidation of methionine to methionine sulfoxide occurs under conditions of cellular oxidative stress, and modulates the function of a diverse array of proteins. Enzymatic systems that install and reverse the methionine sulfoxide modifications have been characterized, however, little is known about potential readers of this oxidative modification. Here, we apply a peptide-crosslinking approach to identify proteins that are able to differentially interact with reduced and oxidized methionine-containing peptides. Specifically, we generated a photo-crosslinking peptide derived from actin, which contains two sites of methionine oxidation, M44 and M47. Our proteomic studies identified heat shock proteins, including HSPA8, as selective for the reduced methionine-containing peptide, whereas the phosphofructokinase isoform, PFKL, preferentially interacts with the oxidized form. We then demonstrate that the favored interaction of PFKL with oxidized methionine is also observed in the full-length actin protein, suggesting a role of methionine oxidation in regulating the actin-PFKL interaction in cells. Our studies demonstrate the potential to identify proteins that can differentiate between reduced and oxidized methionine and thereby mediate downstream protein functions under conditions of oxidative stress. Furthermore, given that numerous sites of methionine oxidation have now been identified, these studies set the stage to identify putative readers of methionine oxidation on other protein targets.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Methionine residues around phosphorylation sites are preferentially oxidized in vivo under stress conditions.Sci Rep. 2017 Jan 12;7:40403. doi: 10.1038/srep40403. Sci Rep. 2017. PMID: 28079140 Free PMC article.
-
Taurine chloramine-induced inactivation of cofilin protein through methionine oxidation.Free Radic Biol Med. 2014 Oct;75:84-94. doi: 10.1016/j.freeradbiomed.2014.07.018. Epub 2014 Jul 22. Free Radic Biol Med. 2014. PMID: 25058340
-
The Oxidized Protein Repair Enzymes Methionine Sulfoxide Reductases and Their Roles in Protecting against Oxidative Stress, in Ageing and in Regulating Protein Function.Antioxidants (Basel). 2018 Dec 12;7(12):191. doi: 10.3390/antiox7120191. Antioxidants (Basel). 2018. PMID: 30545068 Free PMC article. Review.
-
Are Methionine Sulfoxide-Containing Proteins Related to Seed Longevity? A Case Study of Arabidopsisthaliana Dry Mature Seeds Using Cyanogen Bromide Attack and Two-Dimensional-Diagonal Electrophoresis.Plants (Basel). 2022 Feb 21;11(4):569. doi: 10.3390/plants11040569. Plants (Basel). 2022. PMID: 35214905 Free PMC article.
-
Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1.Antioxid Redox Signal. 2015 Oct 1;23(10):814-22. doi: 10.1089/ars.2015.6385. Epub 2015 Jul 16. Antioxid Redox Signal. 2015. PMID: 26181576 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

