H-bond cooperativity: polarisation effects on secondary amides
- PMID: 36320903
- PMCID: PMC9580511
- DOI: 10.1039/d2sc04271a
H-bond cooperativity: polarisation effects on secondary amides
Abstract
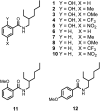
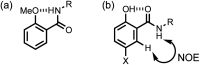
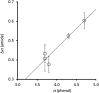
Formation of a H-bond with an amide carbonyl oxygen atom increases the strength of subsequent H-bonds formed by the amide NH, due to polarisation of the bond. The magnitude of this effect has been quantified by measuring association constants for the formation of 1 : 1 complexes of 2-hydroxylbenzamides with tri-n-butyl phosphine oxide. In 2-hydroxybenzamides, there is an intramolecular H-bond between the phenol OH group and the carbonyl oxygen atom. Comparison of the association constants measured for compounds with and without the 2-hydroxy group allows direct quantification of the effect of the intramolecular H-bond on the H-bond donor properties of the amide NH group. Substituents were used to modulate the strength of the intramolecular and intermolecular H-bonds. The presence of an intramolecular H-bond increases the strength of the intermolecular H-bond by more than one order of magnitude in n-octane solution. The increase in the H-bond donor parameter used to describe the amide NH group is directly proportional to the H-bond donor parameter of the phenol OH group that makes the intramolecular H-bond. These polarisation effects will lead to substantial cooperativity in complex systems that feature networks of non-covalent interactions, and the measurements described here provide a quantitative basis for understanding such phenomena.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


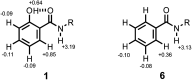



References
LinkOut - more resources
Full Text Sources

