Substrate Ablation by Multivein, Multiballoon Coronary Venous Ethanol for Refractory Ventricular Tachycardia in Structural Heart Disease
- PMID: 36321460
- PMCID: PMC9712228
- DOI: 10.1161/CIRCULATIONAHA.122.060882
Substrate Ablation by Multivein, Multiballoon Coronary Venous Ethanol for Refractory Ventricular Tachycardia in Structural Heart Disease
Erratum in
-
Correction to: Substrate Ablation by Multivein, Multiballoon Coronary Venous Ethanol for Refractory Ventricular Tachycardia in Structural Heart Disease.Circulation. 2023 Jan 10;147(2):e31. doi: 10.1161/CIR.0000000000001118. Epub 2023 Jan 9. Circulation. 2023. PMID: 36622910 No abstract available.
Abstract
Background: Ablation of ventricular tachycardia (VT) in the setting of structural heart disease often requires extensive substrate elimination that is not always achievable by endocardial radiofrequency ablation. Epicardial ablation is not always feasible. Case reports suggest that venous ethanol ablation (VEA) through a multiballoon, multivein approach can lead to effective substrate ablation, but large data sets are lacking.
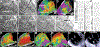
Methods: VEA was performed in 44 consecutive patients with ablation-refractory VT (ischemic, n=21; sarcoid, n=3; Chagas, n=2; idiopathic, n=18). Targeted veins were selected by mapping coronary veins on the epicardial aspect of endocardial scar (identified by bipolar voltage <1.5 mV), using venography and signal recording with a 2F octapolar catheter or by guidewire unipolar signals. Epicardial mapping was performed in 15 patients. Vein segments in the epicardial aspect of VT substrates were treated with double-balloon VEA by blocking flow with 1 balloon while injecting ethanol through the lumen of the second balloon, forcing (and restricting) ethanol between balloons. Multiple balloon deployments and multiple veins were used as needed. In 22 patients, late gadolinium enhancement cardiac magnetic resonance imaged the VEA scar and its evolution.
Results: Median ethanol delivered was 8.75 (interquartile range, 4.5-13) mL. Injected veins included interventricular vein (6), diagonal (5), septal (12), lateral (16), posterolateral (7), and middle cardiac vein (8), covering the entire range of left ventricular locations. Multiple veins were targeted in 14 patients. Ablated areas were visualized intraprocedurally as increased echogenicity on intracardiac echocardiography and incorporated into 3-dimensional maps. After VEA, vein and epicardial ablation maps showed elimination of abnormal electrograms of the VT substrate. Intracardiac echocardiography demonstrated increased intramural echogenicity at the targeted region of the 3-dimensional maps. At 1 year of follow-up, median of 314 (interquartile range, 198-453) days of follow-up, VT recurrence occurred in 7 patients, for a success of 84.1%.
Conclusions: Multiballoon, multivein intramural ablation by VEA can provide effective substrate ablation in patients with ablation-refractory VT in the setting of structural heart disease over a broad range of left ventricular locations.
Keywords: ablation techniques; arrhythmias, cardiac; ethanol; heart ventricles; veins.
Conflict of interest statement
Figures






References
-
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ and Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e190–e252. - PubMed
-
- Arenal A, Avila P, Jimenez-Candil J, Tercedor L, Calvo D, Arribas F, Fernandez-Portales J, Merino JL, Hernandez-Madrid A, Fernandez-Aviles FJ and Berruezo A. Substrate Ablation vs Antiarrhythmic Drug Therapy for Symptomatic Ventricular Tachycardia. J Am Coll Cardiol. 2022;79:1441–1453. - PubMed
-
- Tung R, Xue Y, Chen M, Jiang C, Shatz DY, Besser S, Hu H, Chung FP, Nakahara S, Kim YH, Satomi K, Shen L, Liang E, Liao H, Gu K, Jiang R, Jiang J, Hori Y, Choi JI, Ueda A, Komatsu Y, Kazawa S, Soejima K, Chen SA, Nogami A, Yao Y and Investigators P-S. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent with Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation. 2022. - PubMed
-
- Della Bella P, Baratto F, Vergara P, Bertocchi P, Santamaria M, Notarstefano P, Calo L, Orsida D, Tomasi L, Piacenti M, Sangiorgio S, Pentimalli F, Pruvot E, de Sousa J, Sacher F, Tritto M, Rebellato L, Deneke T, Romano SA, Nesti M, Gargaro A, Giacopelli D, Peretto G and Radinovic A. Does Timing of Ventricular Tachycardia Ablation Affect Prognosis in Patients With an Implantable Cardioverter Defibrillator? Results From the Multicenter Randomized PARTITA Trial. Circulation. 2022. - PubMed
-
- Kumar S, Tedrow UB and Stevenson WG. Adjunctive Interventional Techniques When Percutaneous Catheter Ablation for Drug Refractory Ventricular Arrhythmias Fail: A Contemporary Review. Circ Arrhythm Electrophysiol. 2017;10:e003676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

