AbobotulinumtoxinA is effective in patients with urinary incontinence due to neurogenic detrusor overactivity regardless of spinal cord injury or multiple sclerosis etiology: Pooled analysis of two phase III randomized studies (CONTENT1 and CONTENT2)
- PMID: 36321799
- PMCID: PMC10092111
- DOI: 10.1002/nau.25062
AbobotulinumtoxinA is effective in patients with urinary incontinence due to neurogenic detrusor overactivity regardless of spinal cord injury or multiple sclerosis etiology: Pooled analysis of two phase III randomized studies (CONTENT1 and CONTENT2)
Abstract
Background: Neurogenic detrusor overactivity incontinence (NDOI) is often inadequately managed with oral therapy.
Objective: To assess efficacy and safety of abobotulinumtoxinA (aboBoNT-A; Dysport®; Ipsen Ltd.) according to etiology of NDOI.
Design, setting, and participants: Two phase III, randomized, double-blind studies (CONTENT1 [NCT02660138] conducted in Asia, Europe and North America; CONTENT2 [NCT02660359] conducted in the Americas, Asia, Europe and Oceania) both included patients with spinal cord injury (SCI) or multiple sclerosis (MS), with inadequately managed NDOI, regularly performing clean intermittent catheterization (CIC).
Intervention: Patients in CONTENT1 and CONTENT2 received aboBoNT-A injections 600 U (n = 162)/800 U (n = 161), or placebo (n = 162) into the detrusor muscle.
Outcome measurements and statistical analysis: Primary endpoint: mean change from baseline in number of NDOI episodes/week at Week 6. Secondary endpoints: proportion of patients with no NDOI episodes; incontinence-related quality of life (I-QoL); urodynamic parameters; and time-to-retreatment. Safety was also assessed. Statistical analyses were conducted for pooled populations by etiology (aboBoNT-A doses vs. placebo).
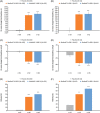
Results and limitations: Of 485 randomized patients, 341 (70%) and 144 (30%) had SCI and MS etiologies, respectively. A significant reduction was observed in mean NDOI episodes/week at Week 6 with both aboBoNT-A doses versus placebo in the SCI (all p < 0.001) and MS (all p < 0.01) groups, as well as significant improvements in I-QoL and urodynamic parameters. Median time-to-retreatment was longer in patients with MS (48-62 weeks across doses) than those with SCI (39-44 weeks). Safety data were similar between etiologies. Urinary tract infection was the most frequent adverse event; similar numbers were reported across treatment groups.
Conclusions: AboBoNT-A was well tolerated and significantly improved continence and bladder function, and QoL, in patients with SCI or MS with NDOI performing regular CIC.
Patient summary: AboBoNT-A injections improved QoL, symptoms, and bladder function in patients with SCI or MS with bladder muscle overactivity that causes incontinence.
Keywords: AbobotulinumtoxinA; Botulinum toxin; multiple sclerosis; neurogenic detrusor overactivity incontinence; spinal cord injury.
© 2022 The Authors. Neurourology and Urodynamics published by Wiley Periodicals LLC.
Conflict of interest statement
Pierre Denys: Consultancy: Allergan, Taris, Medtronic, Coloplast. Grant research study: Ipsen and Allergan. Juan Carlos Castaño Botero: Astellas (consultant and lecturer fees), Boston Scientific (consultant and lecturer fees) Medtronic (consultant fees, trial investigator), Ipsen (trial investigator). Ricardo Luis Vita Nunes: Grant/Research study: Ipsen; Consultancy: Astellas Pharma, Zambon; Lectures: Astellas, Zambon, Zodiac, Aché, Coloplast. Barton Wachs: Nothing to disclose. Cristiano Mendes Gomes: Grant/Research study: Ipsen; Consultancy: Astellas Pharma, Boston Scientific; Lectures: Astellas, Boston Scientific, Zodiac. Grigory Krivoborodov: Lectures: Astellas, Coloplast, Pierre Fabre, Braun, Medtronic. Le Mai Tu: Consultancy and Lectures: Astellas Pharma, Pfizer. Giulio Del‐Popolo: Grant/Research study: Ipsen, Wellspect, B Braun, Hollister; Consultant: Coloplast, Wellspect, Pierre Fabre, Braun. Catherine Thompson: Employee of June Pharma under contract by Ipsen. Claire Vilain and Magali Volteau: Employee of Ipsen. Michael Kennelly: Research Grants: Allergan, Amphora, Axonics, Boston Scientific, Coloplast, Cook Myosite, Dignify Therapeutics, Ipsen, Taris, Uro1, FemPulse, EBT Medical; Consultancy Fees: Allergan, Boston Scientific, Coloplast, Laborie, Urovant.
Figures


References
-
- Gajewski JB, Schurch B, Hamid R, et al. An International Continence Society (ICS) report on the terminology for adult neurogenic lower urinary tract dysfunction (ANLUTD). Neurourol Urodyn. 2018;37(3):1152‐1161. - PubMed
-
- Haab F. Chapter 1: the conditions of neurogenic detrusor overactivity and overactive bladder. Neurourol Urodyn. 2014;33(Suppl 3):S2‐S5. - PubMed
-
- Biardeau X, Corcos J. Intermittent catheterization in neurologic patients: update on genitourinary tract infection and urethral trauma. Ann Phys Rehabil Med. 2016;59(2):125‐129. - PubMed
-
- Stöhrer M, Blok B, Castro‐Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56(1):81‐88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

