A Cristae-Like Microcompartment in Desulfobacterota
- PMID: 36321837
- PMCID: PMC9764997
- DOI: 10.1128/mbio.01613-22
A Cristae-Like Microcompartment in Desulfobacterota
Abstract
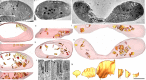
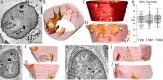
Some Alphaproteobacteria contain intracytoplasmic membranes (ICMs) and proteins homologous to those responsible for the mitochondrial cristae, an observation which has given rise to the hypothesis that the Alphaproteobacteria endosymbiont had already evolved cristae-like structures and functions. However, our knowledge of microbial fine structure is still limited, leaving open the possibility of structurally homologous ICMs outside the Alphaproteobacteria. Here, we report on the detailed characterization of lamellar cristae-like ICMs in environmental sulfate-reducing Desulfobacterota that form syntrophic partnerships with anaerobic methane-oxidizing (ANME) archaea. These structures are junction-bound to the cytoplasmic membrane and resemble the form seen in the lamellar cristae of opisthokont mitochondria. Extending these observations, we also characterized similar structures in Desulfovibrio carbinolicus, a close relative of the magnetotactic D. magneticus, which does not contain magnetosomes. Despite a remarkable structural similarity, the key proteins involved in cristae formation have not yet been identified in Desulfobacterota, suggesting that an analogous, but not a homologous, protein organization system developed during the evolution of some members of Desulfobacterota. IMPORTANCE Working with anaerobic consortia of methane oxidizing ANME archaea and their sulfate-reducing bacterial partners recovered from deep sea sediments and with the related sulfate-reducing bacterial isolate D. carbinolicus, we discovered that their intracytoplasmic membranes (ICMs) appear remarkably similar to lamellar cristae. Three-dimensional electron microscopy allowed for the novel analysis of the nanoscale attachment of ICMs to the cytoplasmic membrane, and these ICMs are structurally nearly identical to the crista junction architecture seen in metazoan mitochondria. However, the core junction-forming proteins must be different. The outer membrane vesicles were observed to bud from syntrophic Desulfobacterota, and darkly stained granules were prominent in both Desulfobacterota and D. carbinolicus. These findings expand the taxonomic breadth of ICM-producing microorganisms and add to our understanding of three-dimensional microbial fine structure in environmental microorganisms.
Keywords: Desulfobacterota; anaerobic oxidation of methane; compartmentalization; electron microscope tomography; intracytoplasmic membrane; outer membrane vesicles; pera; pera junction; sulfate reduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pinevich AV. 1997. Intracytoplasmic membrane structures in bacteria. Endocytobiosis Cell Res:9–40.
-
- Tucker JD, Siebert CA, Escalante M, Adams PG, Olsen JD, Otto C, Stokes DL, Hunter CN. 2010. Membrane invagination in Rhodobacter sphaeroides is initiated at curved regions of the cytoplasmic membrane, then forms both budded and fully detached spherical vesicles. Mol Microbiol 76:833–847. doi:10.1111/j.1365-2958.2010.07153.x. - DOI - PubMed
