Ibuprofen: a weak inhibitor of carbonic anhydrase II
- PMID: 36322425
- PMCID: PMC9629514
- DOI: 10.1107/S2053230X22009761
Ibuprofen: a weak inhibitor of carbonic anhydrase II
Abstract
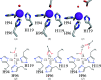
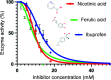
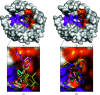
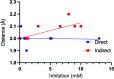
Carbonic anhydrases (CAs) are drug targets for a variety of diseases. While many clinically relevant CA inhibitors are sulfonamide-based, novel CA inhibitors are being developed that incorporate alternative zinc-binding groups, such as carboxylic acid moieties, to develop CA isoform-specific inhibitors. Here, the X-ray crystal structure of human CA II (hCA II) in complex with the carboxylic acid ibuprofen [2-(4-isobutylphenyl)propanoic acid, a common over-the-counter nonsteroidal anti-inflammatory drug] is reported to 1.54 Å resolution. The binding of ibuprofen is overlaid with the structures of other carboxylic acids in complex with hCA II to compare their inhibition mechanisms by direct or indirect (via a water) binding to the active-site zinc. Additionally, enzyme-inhibition assays using ibuprofen, nicotinic acid and ferulic acid were performed with hCA II to determine their IC50 values and were compared with those of other carboxylic acid binders. This study discusses the potential development of CA inhibitors utilizing the carboxylic acid moiety.
Keywords: X-ray crystallography; carbonic anhydrases; carboxylic acid-based inhibitors; ibuprofen; kinetics.
Figures

Similar articles
-
Carbonic anhydrase II in complex with carboxylic acid-based inhibitors.Acta Crystallogr F Struct Biol Commun. 2019 Mar 1;75(Pt 3):166-170. doi: 10.1107/S2053230X18018344. Epub 2019 Feb 20. Acta Crystallogr F Struct Biol Commun. 2019. PMID: 30839290 Free PMC article.
-
5-Substituted-(1,2,3-triazol-4-yl)thiophene-2-sulfonamides strongly inhibit human carbonic anhydrases I, II, IX and XII: solution and X-ray crystallographic studies.Bioorg Med Chem. 2013 Sep 1;21(17):5130-8. doi: 10.1016/j.bmc.2013.06.041. Epub 2013 Jun 27. Bioorg Med Chem. 2013. PMID: 23859774
-
Insights into the effect of elaborating coumarin-based aryl enaminones with sulfonamide or carboxylic acid functionality on carbonic anhydrase inhibitory potency and selectivity.Bioorg Chem. 2022 Sep;126:105888. doi: 10.1016/j.bioorg.2022.105888. Epub 2022 May 25. Bioorg Chem. 2022. PMID: 35661530
-
Carbonic anhydrase inhibitors: X-ray crystal structure of a benzenesulfonamide strong CA II and CA IX inhibitor bearing a pentafluorophenylaminothioureido tail in complex with isozyme II.Bioorg Med Chem Lett. 2005 Apr 1;15(7):1937-42. doi: 10.1016/j.bmcl.2005.01.086. Bioorg Med Chem Lett. 2005. PMID: 15780637
-
Carbonic anhydrase inhibitors: X-ray and molecular modeling study for the interaction of a fluorescent antitumor sulfonamide with isozyme II and IX.J Am Chem Soc. 2006 Jun 28;128(25):8329-35. doi: 10.1021/ja061574s. J Am Chem Soc. 2006. PMID: 16787097
Cited by
-
Allura Red AC is a xenobiotic. Is it also a carcinogen?Carcinogenesis. 2024 Oct 10;45(10):711-720. doi: 10.1093/carcin/bgae057. Carcinogenesis. 2024. PMID: 39129647 Free PMC article. Review.
References
-
- Berman, H. M., Henrick, K. & Nakamura, H. (2003). Nat. Struct. Mol. Biol. 10, 980. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

